Quantum number l formula ~ M 2 l 1 values of l 1 to -1 including zero. A term symbol of the free ion takes the form 2S 1 L where L is the total orbital angular momentum quantum number and S is the total intrinsic spin quantum number. Indeed recently is being hunted by consumers around us, maybe one of you personally. People are now accustomed to using the net in gadgets to view video and image data for inspiration, and according to the name of this post I will talk about about Quantum Number L Formula For d-orbital ℓ 2.
Source Image @ www.priyamstudycentre.com
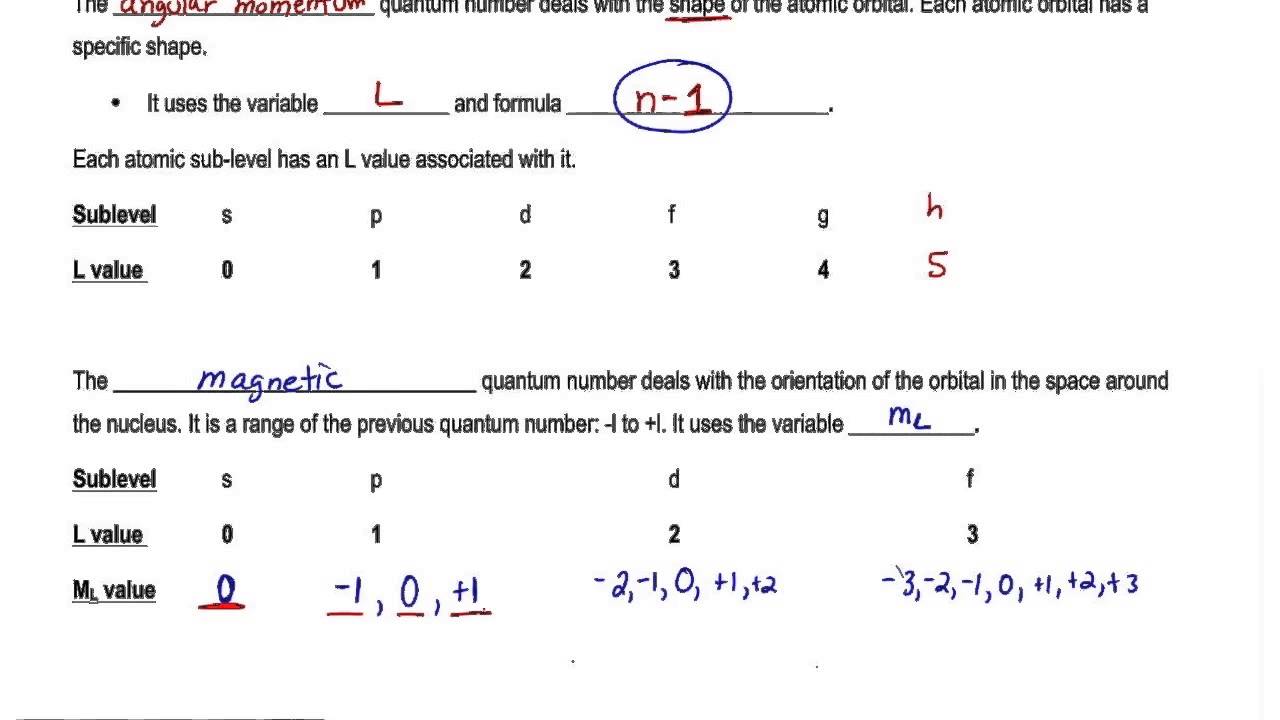
Quantum Number Orbital Definition Formula Diagram Shape

L 0 ml 0 orbital s. The. Your Quantum number l formula image are ready in this website. Quantum number l formula are a topic that has been searched for and liked by netizens today. You can Get or bookmark the Quantum number l formula files here
Quantum number l formula - As the symbol suggests it has to do with l the angular momentum quantum number. Angular momentum quantum number l n 1. What does the Magnetic Quantum Number Determine. For p-orbita ℓ 1.
We used the formula n2 to calculate the total number of orbitals possible at the n at any value that is for n 3. Magnetic quantum number ml 2 1 0 1 2. They can even take on more complex shapes as the value of the angular quantum number becomes larger. Orbitals within the shells are divided into.
What is the formula of principal quantum number. Values of are from zero to n-1. If n 3 l can be either 0 1 or 2. The angular quantum number l can be any integer between 0 and n - 1.
A n 5 l 0 ml 0 ms -12 B n 4 l 1 ml 0 ms -12 C n 5 l What is the probability that the state 0175 eV above the Fermi level is occupied at 300 K. For a solution of either the nonrelativistic Pauli equation or the relativistic Dirac equation the quantized angular momentum see angular momentum quantum number can be written as. This is a component of the atomic electrons total orbital angular momentum whose magnitude is related to the azimuthal quantum number of its subshell by the equation. The value 2S 1 is called the multiplicity.
Azimuthal quantum number describes the shape of orbital. Give the possible values of n l and ml for this electron. Therefore if the value of n 3 3The quantum number l can have values from 0 1 2. Orbitals that have same value of principal quantum number form a Shelln.
Thus the physically accepted values of land mare L 0 1. The secondary quantum number divides the shells into smaller groups of orbitals called subshells sublevels. Specifies the shape of an orbital with a particular principal quantum number. L ℏ ℓ ℓ 1 displaystyle Lhbar sqrt ell ell 1.
Where ħ is the reduced Plancks constant L 2 is the orbital angular momentum operator and is the wavefunction of the electron. N 2 l 1 m l 0 m s. S s s 1 ℏ displaystyle Vert mathbf s Vert sqrt ss1hbar. For s-orbital ℓ 0.
In order for Rodrigues formula to make sense l must be non-negative integer. For the 3d orbital Principal quantum number n 3. An electron is in one of the 3d orbitals. L 0 n-1.
For the value of l. Angular Momentum Secondary Azimunthal Quantum Number l. Usually a letter code is used to identify l to avoid confusion with n. The magnetic quantum number primarily determines the number of orbitals and the orientation of orbitals in a given sub-shell.
The fourth quantum number is independent of the first three allowing the first three quantum numbers of two electrons to be the same. The principal quantum is calculated as the number of the shell rmn The azimuthal quantum number is calculated by rml n-1 The magnetic quantum number is calculated by rmm -l to rml The spin quantum number has two values only 12 and 12. L describes the shape of the orbital. An atomic electrons angular momentum L is related to its quantum number ℓ by the following equation.
Orbitals have shapes that are best described as spherical l 0 polar l 1 or cloverleaf l 2. 32 33 9. How are quantum numbers calculated. Ml is the magnetic quantum number corresponding to the projection of the angular momentum of an orbital ie.
For f-orbital ℓ 3. Magnetic quantum number m. If l 2 m can be -2 -1 0 1 or 2. Its orientation in space.
And this is because l n-1. It is denoted by. The magnetic quantum number can be derived from solving the azimuthal equation of the hydrogen Schrodinger equation. L w Plw.
Each l value represents a specific orbit named after the description of the hydrogen spectrum such s for sharp l 0 p for principal l 1 d for diffuse l 2 f for fundamental l 3 etc. With the help of the value of azimuthal quantum number we can determine the total number of energy sub-levels in a given energy level. Kathryn Haas Exercise 1121. Consequently it is dependent on the orbital angular momentum.
Since the spin can be 12 or 12 there are two combinations. The angular quantum number l describes the shape of the orbital. Lets look at various values of l and their corresponding ml. Moreover if m l then Equation 31 implies Pm l 0.
Azimuthal quantum number l 2. The magnetic quantum number m can be any integer between -l and l. The total number of orbitals possible at the n 3 energy level is 9. It describes orientation of orbital in space under magnetic field which obtained due to angular momentum of electron and thus it relates to the value of l.
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ study.com
Source Image @ www.youtube.com
Source Image @ www.youtube.com
Source Image @ slideplayer.com
Source Image @ study.com
Source Image @ study.com
Source Image @ www.khanacademy.org
Source Image @ study.com
If you re searching for Quantum Number L Formula you've come to the right place. We ve got 10 images about quantum number l formula including pictures, pictures, photos, backgrounds, and more. In these web page, we also provide number of images available. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.
If the publishing of this site is beneficial to your suport by revealing article posts of this site to social media accounts as such as for example Facebook, Instagram and others or may also bookmark this website page with all the title Magnetic Quantum Number Definition Example Video Lesson Transcript Study Com Make use of Ctrl + D for laptop or computer devices with Glass windows operating system or Order + D for computer devices with operating system from Apple. If you use a smartphone, you can even use the drawer menu on the browser you use. Be it a Windows, Mac pc, iOs or Google android operating-system, you'll be able to download images using the download button.







0 comments:
Post a Comment