Empirical formula of science ~ Na 23 To gain full marks you must show all your working. An empirical formula is one that shows the lowest whole-number ratio of the elements in a compound. Indeed recently has been hunted by consumers around us, perhaps one of you. Individuals are now accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this post I will talk about about Empirical Formula Of Science The concept of empirical formula in the field of chemistry is very useful in finding out the elemental ratios of compounds that are similar in composition.
Source Image @ chemistrygod.com
Understanding Chemistry Formulas Chemistrygod

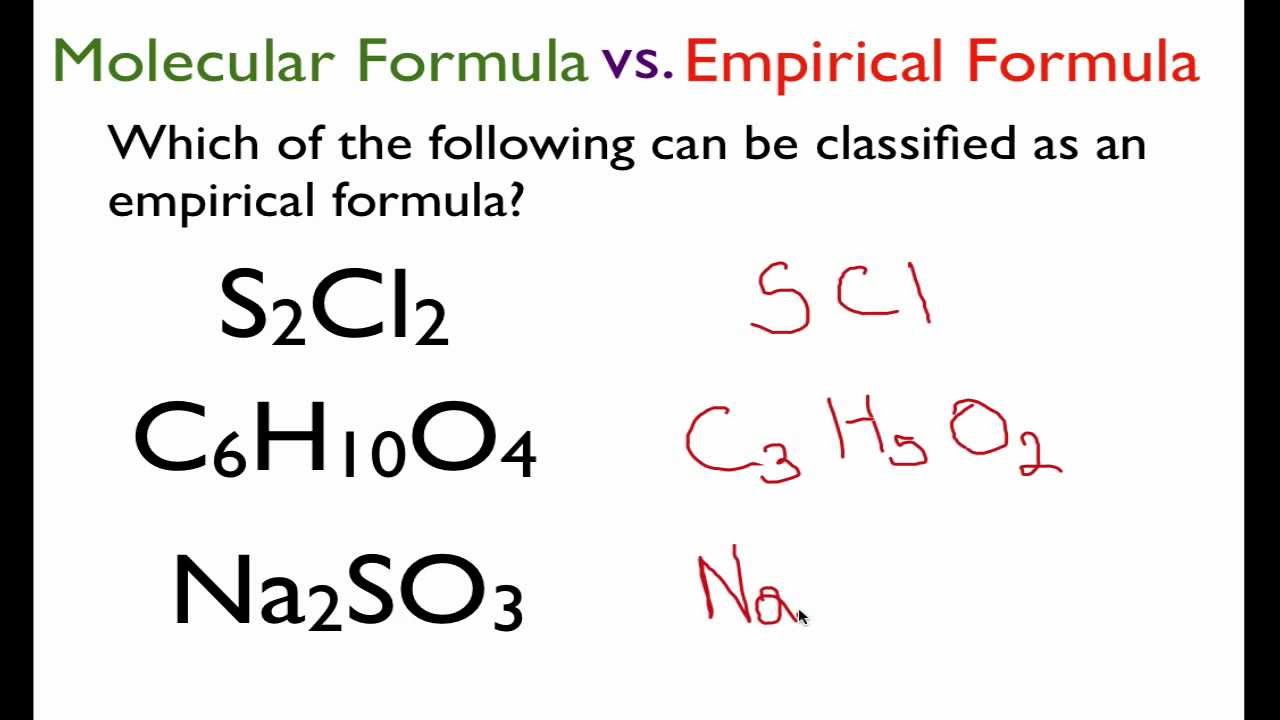
In chemistry the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. Finally convert numbers to the whole numbers. Your Empirical formula of science pictures are available in this site. Empirical formula of science are a topic that has been hunted for and liked by netizens today. You can Get or bookmark the Empirical formula of science files here
Empirical formula of science - The simplest formula or the empirical formula provides the lowest whole number ratio of atoms existent in a compound. The empirical formula is a chemical formula that represents the simplest ratio of atoms in the chemical formula of the compound. The ratios are denoted by subscripts next to the element symbols. The non-whole number empirical formula of the compound is Fe 1 O 15.
Divide by fractional component of each mole value. The empirical formula N1 was fitted directly based on the summarized experimental dataset which was in contrast to the augmented empirical formula N2. The relative number of atoms of every element in the compound is provided by this formula. An empirical formula consists of symbols representing elements in a compound such as Na for sodium and Cl for chlorine and subscripts indicating the relative number of atoms of each constituent element.
For example in hydrogen peroxide there is one part by mass of hydrogen for every 16 parts by mass of oxygen. A compound contains 8879 oxygen element and 1119 hydrogen element. Other articles where empirical formula is discussed. The empirical formula is the simplest type of chemical formula as it shows the relative number of atoms of each element in a given compound.
Scientists found that a compound contained. So if we assume a ratio of two chlorine atoms for every one mercury atom the likely empirical formula is for every mercury atom we will have two chlorines. It is determined using data from experiments and. Empirical formula C 6 H 11 NO.
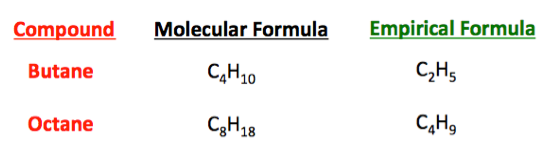
The empirical formula of a compound is defined as the formula that shows the ratio of elements present in the compound but not the actual numbers of atoms found in the molecule. In your case you have C12H22O11 as the empirical formula because the ratio that exists between carbon C hydrogen H and oxywen O is the smallest whole number ratio that can exist for this formula. Examples of empirical formula The molecular formula of ethane is C 2 H 6. Because the structure of ionic compounds is an extended three-dimensional network of positive and negative ions all formulas of ionic compounds are empirical.
And so this could be the likely empirical formula. The relation between Empirical formula and the molecular formula is given by. Compute the empirical formula of this compound. Therefore for every hydrogen atom there is one oxygen atom and the empirical formula is H-O.
Along the other way a ML surrogate model Ms f p i was built by the use of physical and chemical features p i of the elements as this is known to be closely related to the transformation temperature. Solved Examples on Empirical Formula. In other words you can divide 12 22 and 11 by a whole number and still get whole numbers for all three elements. A simple example of this concept is that the empirical formula of sulfur monoxide or SO would simply be SO as is the empirical formula of disulfur dioxide.
It can be used as a standard value when several ionic compounds are compared regarding their atomic structure chemical properties and percentage of elemental composition. The empirical formula for a molecule is the lowest whole number ratio of atoms the molecular formula can be broken down into. Where n is a whole number and is a ratio of molecular mass to empirical formula mass. Empirical formula.
Multiply each of the moles by the smallest whole number that will convert each into a whole number. Thus the empirical formula mass of glucose is 12 x 1 1 x 2 16 x 1 30. It can also be the molecular formula which gives the number of. Of a substance is the simplest whole number ratio.
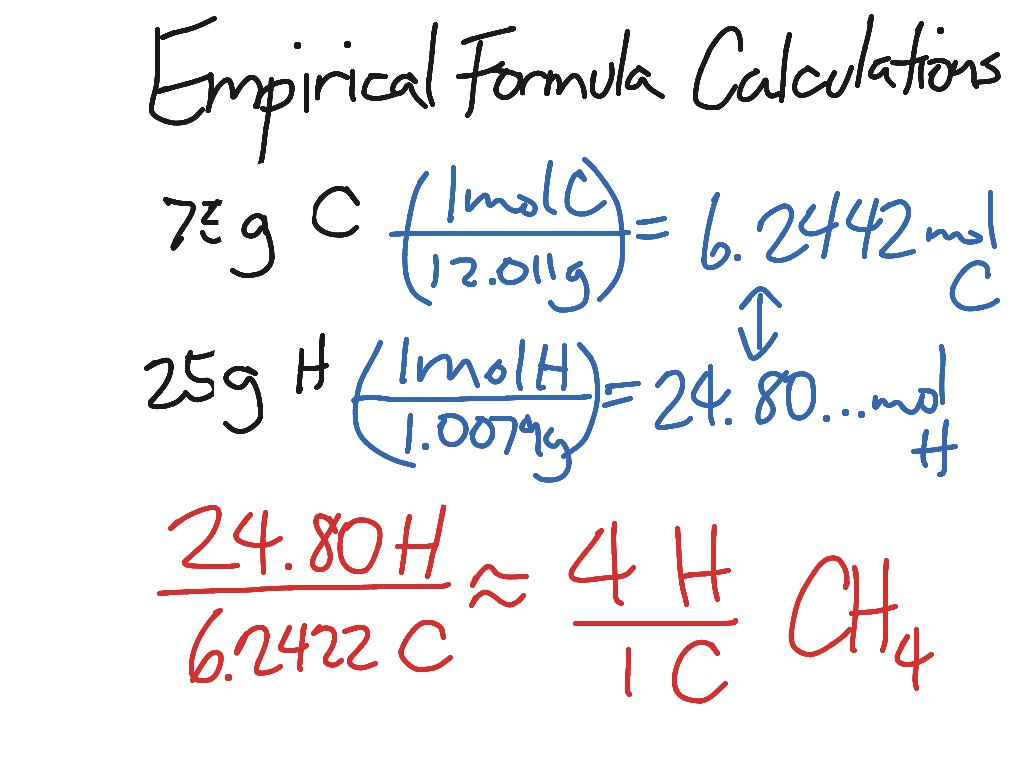
The empirical formula is the simplest whole-number ratio of atoms in a compound. Since the moles of O is still not a whole number both moles can be multiplied by 2 while rounding to a whole number. Use the percentages to calculate the empirical formula of the compound. Relative atomic masses A r.
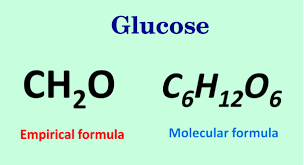
For example consider sugar whose molecular formula is Ceq_6 eqH. This set of whole numbers will be the subscripts in the empirical formula. Empirical formulae The empirical formula of a compound is the simplest whole number ratio of atoms of each element in the compound. The empirical formula for glucose is CH 2 O.
FeO 2 115 23. A subscript is not used however unless the number is more than one. Molecular Formula textn times Empirical Formula is the general relationship between the empirical and molecular formulas. Molecular formula n x Empirical formula.
1 Find the empirical formula of a compound that has 467 of Oxygen O 533 of Silicon Si. R times whole number Empirical Formula.
Source Image @ study.com
Source Image @ www.youtube.com
Source Image @ www.showme.com
Source Image @ socratic.org
Source Image @ twitter.com
Source Image @ sciencenotes.org
Source Image @ www.scienceinschool.org
Source Image @ study.com
Source Image @ thefactfactor.com
If you are looking for Empirical Formula Of Science you've arrived at the ideal location. We have 10 images about empirical formula of science adding pictures, pictures, photos, wallpapers, and more. In these page, we also have variety of graphics available. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.
If the publishing of this webpage is beneficial to your suport by spreading article posts of the site to social media accounts that you have got such as Facebook, Instagram among others or may also bookmark this blog page with all the title Empirical Formula And Molecular Formula Meaning And Its Determination Make use of Ctrl + D for pc devices with Home windows operating system or Command word + D for computer devices with operating system from Apple. If you are using a smartphone, you can also utilize the drawer menu on the browser you utilize. Be it a Windows, Macintosh personal computer, iOs or Google android operating-system, you'll still be able to download images utilizing the download button.





0 comments:
Post a Comment