Planck's quantum hypothesis formula ~ Particles can only have discrete values of energy. To explain the colors of hot glowing matter he proposed that energy is radiated in very minute and discrete quantized amounts of packets rather than in a continuous unbroken wave. Indeed lately has been searched by consumers around us, perhaps one of you. Individuals are now accustomed to using the internet in gadgets to see video and image data for inspiration, and according to the name of the article I will talk about about Planck's Quantum Hypothesis Formula Plancks quantization of energy is described by his famous equation.
Source Image @ oxscience.com
Planck S Radiation Law Derivation Oxscience
PowerPoint PPT presentation. Plancks law is a pioneering result of modern physics and quantum theory. Your Planck's quantum hypothesis formula photos are ready in this website. Planck's quantum hypothesis formula are a topic that has been searched for and liked by netizens now. You can Find and Download or bookmark the Planck's quantum hypothesis formula files here
Planck's quantum hypothesis formula - This is the famous formula from Planck for a black bodys energy density. The StefanBoltzmann law determines the total blackbody emissive power E b which is the sum of the radiation emitted over all wavelengths. First we consider the properties of waves in. Plancks Hypothesis Energy per frequency.
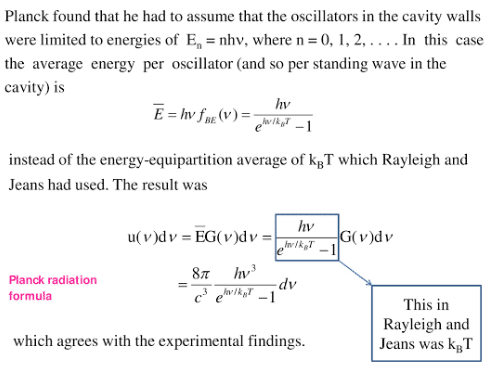
Planck Radiation Formula From the assumption that the electromagnetic modes in a cavity were quantized in energy with the quantum energy equal to Plancks constant times the frequency Planck derived a radiation formula. The programme to derive this formula is as follows. Eλdλ Number of oscillators per unit volume in the interval λ and λdλ x Average energy per oscillator This equation represents Plancks radiation law in terms of wavelength. For black-body radiation is described by the Planck formula Figure 101 ud 8h3 c3 1 ehkT 1 d 101 where Plancks constant h 6626 1034 J s.
The electron volt is a smaller unit of energy than a joule. ƒ frequency of light Formula associated with Plancks Quantum hypothesis. Plancks work in thermodynamics led to the formulations of his quantum theory. Plancks quantization of energy is described by the his famous equation.
In 1900 Planck reported his discovery of a formula that accurately described the shape of a blackbody spectrum for all wavelengths and temperatures. Plancks Law Plancks Hypothesis. Moller gave a very cumbrous proof 1 of the in-variance of h using plane monochromatic wave function in a lengthy and complicated 4 dimensional formalism and added. Plancks hypothesis that energy is radiated and absorbed in discrete quanta or energy packets precisely matched the observed patterns of blackbody radiation and resolved the ultraviolet catastrophe.
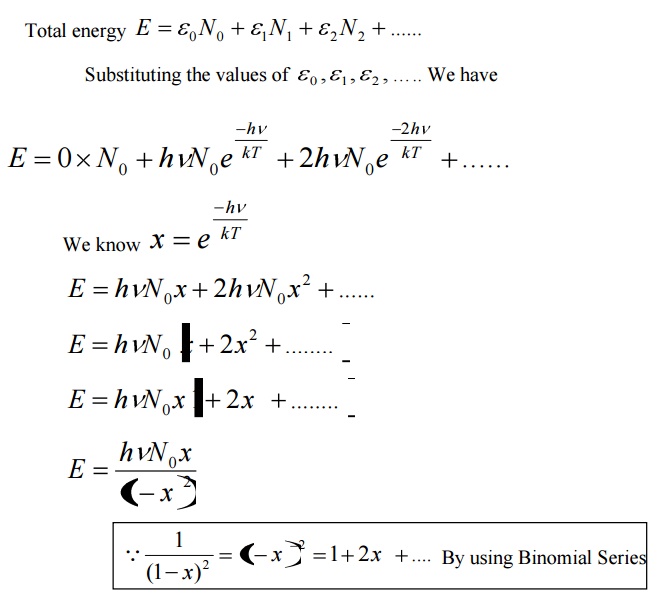
Stay tuned to BYJUS to know more about Plancks law and Plancks quantum theory along with worked problems and solutions. H Plancks constant. E n nhf where n is a positive interger f is the frequency of the oscillating particle h is the Plancks constant. N 1 2 3 4.
Plancks formula for the distribution of energy in the radiation from a black body was the starting point of the quantum theory which has been developed during the last 20 years and has borne a wealth of fruit in the energy domain of physics. The Success of Plancks quantum hypothesis raises the question. 1 eV 16 X 10-19 J. 1 E h v.
Quantum mechanics began with the solution of the problem of blackbody radiation by Plancks quantum hypothesis. When light strikes a surface of a material electrons are emitted. Plancks hypothesis of energy quanta states that the amount of energy emitted by the oscillator is carried by the quantum of radiation Delta E. Blackbody - absorbs all radiation falling on it so that any light observed.
The energy density of radiation between wavelengths λ and λdλ is given by. Where the proportionality constant h is called Plancks constant one of the most accurately known fundamental constants in science. Maybe when a body emits or absorbs light of frequency f it cant emit any old amount of energy it likes because there is some special energy. As an introduction to his reasoning Einstein recapitulated Plancks model of hypothetical resonant material electric oscillators as sources and sinks of radiation but then he offered a new argument disconnected from that model but partly based on a thermodynamic argument of Wien in which Plancks formula ϵ hν played no role.
421 E h ν. Plancks law describes the spectrum of blackbody radiation which depends only on the objects temperature and relates the spectral blackbody emissive power E bλ. In the interaction of light with matter energy can only be exchanged between the light in a cavity and the atoms in the walls of the cavity by the discrete amount. Pler formula is given by vv2 1 cos1ETE 7 From equation 6 and 7 we finally get EvEvh invariant We get Plancks relation Eh Qwhere h is inertial frame invariant.
The particlesoscillators near the surface of the blackbody which emits the blackbody radiation can only have discrete values of energy E n. H Plancks Constant 662times 10-34Js k Boltzmanns Constant 1381 10-23 JK. What actually is the nature of radiation. For Plancks hypothesis doubts the wave nature of it.
A black bodys energy density between λ and λ dλ is the energy of a mode E hc λ times the density of photon states times the probability that the mode is filled. In the mean time the pioneering work of Einstein on photo electricity in 1905 put. This law explained significantly the entire blackbody spectrum. Delta E hf labelplanck Recall that the frequency of electromagnetic radiation is related to its wavelength and to the speed of light by the fundamental relation flambda c.
Where h is a constant now known as Plancks constant h 6626176 x 10 -34 J s Plancks proposal then requires that all the energy in the atomic vibrations with frequency f can be written as. The average energy per mode or quantum is the energy of the quantum times the probability that it will be occupied the Einstein-Bose distribution function. H 66 10-34 J s or J Hz since Hz 1s Plancks quantum hypothesis. Having found an emperical formula that fit the observations he then sought a.
In this lecture we demonstrate why quantum con-cepts are necessary to account for this formula. This law is named after a German theoretical. H 6626070040 81 10 34 J s.
Source Image @ byjus.com
Source Image @ slideplayer.com
Source Image @ www.quora.com
Source Image @ www.slideserve.com
Source Image @ www.facebook.com
Source Image @ www.brainkart.com
Source Image @ www.facebook.com
Source Image @ www.youtube.com
Source Image @ www.youtube.com
If you re searching for Planck's Quantum Hypothesis Formula you've reached the perfect location. We have 10 graphics about planck's quantum hypothesis formula adding pictures, photos, photographs, wallpapers, and much more. In these webpage, we also provide variety of images available. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.
If the publishing of this site is beneficial to your suport by sharing article posts of the site to social media accounts which you have such as for example Facebook, Instagram among others or can also bookmark this blog page using the title Structure Of Atom Class 11 Chemistry Planck S Quantum Theory Of Radiation Youtube Employ Ctrl + D for laptop devices with Glass windows operating system or Command + D for pc devices with operating-system from Apple. If you use a smartphone, you can also utilize the drawer menu of this browser you use. Be it a Windows, Mac pc, iOs or Android os operating-system, you'll still be able to download images utilizing the download button.






0 comments:
Post a Comment