Energy formula quantum mechanics ~ And the expectation value for energy becomes. Or in 1D. Indeed lately has been hunted by consumers around us, perhaps one of you. People are now accustomed to using the internet in gadgets to see video and image information for inspiration, and according to the title of this article I will discuss about Energy Formula Quantum Mechanics When two objects are placed close together they experience a force called the Casimir force that can only be explained by quantum mechanics.
Source Image @ slideplayer.com
Introduction To Quantum Mechanics Ppt Video Online Download
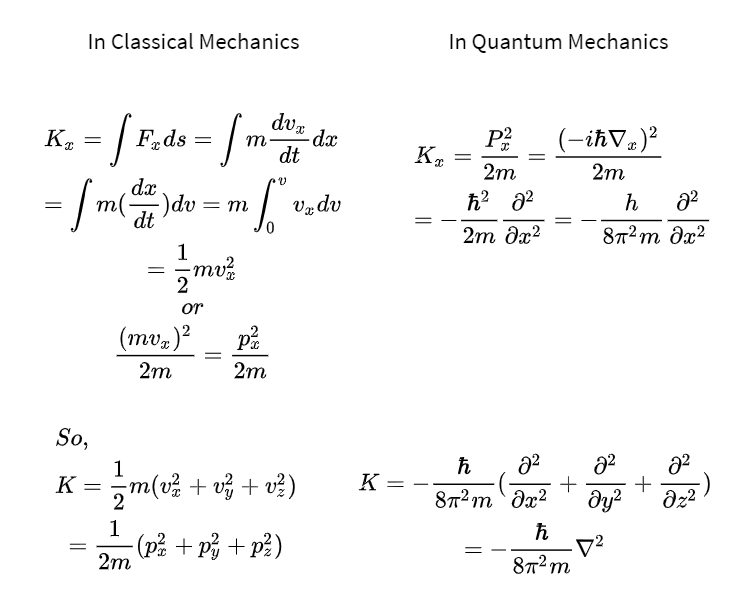
It is a wave equation in terms of the wavefunction which predicts analytically and precisely the probability of events or outcome. So we begin with a lightning review of classical mechanics whose formulation begins but does not end with Newtons law F ma. Your Energy formula quantum mechanics pictures are ready in this website. Energy formula quantum mechanics are a topic that has been searched for and liked by netizens today. You can Find and Download or bookmark the Energy formula quantum mechanics files here
Energy formula quantum mechanics - The energy of the particle in a 3-D cube ie L_xL_y L in the ground state is given by Equation ref3910 with n_x1 n_y1 and n_z1. Eisberg and Resnick Quantum Physics pp 3-24. Specifically this probability is. Quantum number ni to a lower-energy orbit having nf.
In Quantum mechanics the particle is described by a wavefunction xyzt It is related to the probability of finding an electron at time t in a volume dxdydz. I R - -ntZ-ellltrtl 112 The Bohr formula fbr energy levels did not agree as well w ith the observed pattern of emission spectra for species containing more than a single electron. We can now ask about energy but the concept of energy in quantum mechanics is a bit different from what we are used to in classical mechanics. INTRODUCTION TO QUANTUM MECHANICS 1926 Dirac.
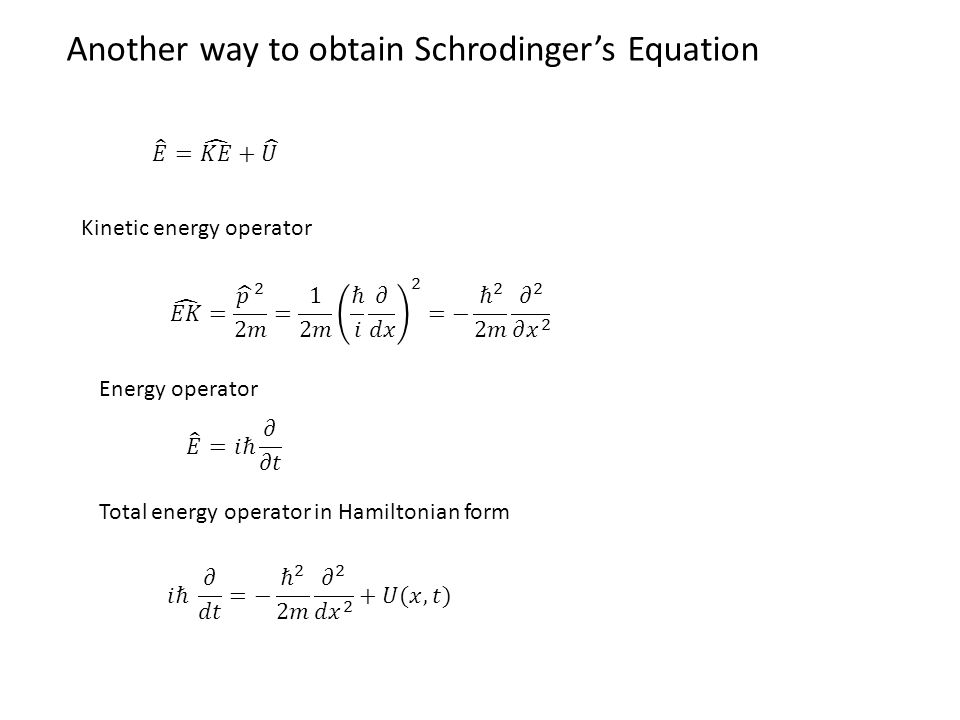
102 The Schrodinger equation In this section well give a derivation of the Schrodinger equation. Here the symbol R is used to denote the fblloing collection of factors. Formula Rydberg formula for hydrogen binding energy quantum number W frac 136 mathrmeV n2. 341 ℏ 2 2 m d 2 ψ x d x 2 E ψ x.
Strictly speaking because Fp is a work rate and K is an energy rate the relation between Fp and K is the work rate-energy rate theorem of quantum mechanics. The term Quantum Mechanics was coined by a group of physicists including Max Born Wolfgang Pauli and Werner Heisenberg in the early 1920s at the University of Göttingen. Although classical mechanics is now regarded as only an approximation to quan-tum mechanics it is still true that much of the structure of the quantum theory is inherited from the classical theory that it replaced. Classically a single particle has a constant energy given by the sum of its potential energy which depends on its position and its kinetic energy which depends on its momentum.
Its the quantum mechanical equation of motion which is postulated. For a particle in one dimension. 1 1. This is important for the un-.
We consider the one-dimensional case with motion only in the x -direction giving the time-independent Schrödinger equation. You can make various plausibility arguments for it eg. The simplest system in quantum mechanics has the potential energy V 0 everywhere. Plancks formula Plancks Theory of Cavity Radiation 1900 energy quantization ref.
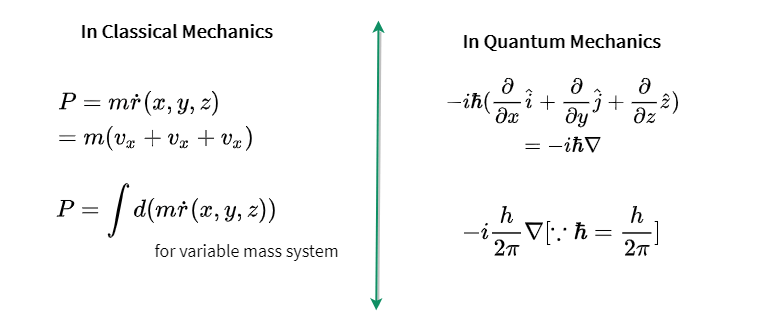
Paul Dirac showed that Heisenbergs and Schrodingers versions of quantum mechanics were equivalent in that they could both be derived from a more general version of quantum mechanics. It defines various principles and theories for studying quantum mechanics and its applications. In general the expectation value for any observable quantity is found by putting the quantum mechanical operator for that observable in the integral of the wavefunction over space. Quantum mechanics is also needed to understand the interaction of photons with materials in solar cells as well as many topics in material science.
X yzt 2dxdydz or dxdydz. The detailed outcome is not strictly determined but given a. Furthermore the amount of energy in one quantum depended on the frequency of the oscillation in fact linearly. Quanta refers to the lowest amount of energy as characterized by quantum theory.
This is called a free particle since it has no forces acting on it. The gradual acknowledgment by scientists that matter has wave-like properties and radiation has. Index Schrodinger equation concepts. This energy E_111 is.
Quantum theory refers to the theoretical study of matter and its properties in terms of atoms protons neutrons electrons etc. Both matter and radiation have characteristics of waves and particles at the fundamental level. Energy is quantized such that Enhν where ninteger and h663x10-34 js. According to Plancks assumption the energy exchanged between an electromagnetic wave of frequency ν and matter can only occur as multiple of hν which he termed energy of a quantum.
From the kinematical relation 2mKṗppṗ and the dynamical relation ṗF we can express the quantum work-energy theorem in the symmetrical form 2mKFppF. Since the energy of a free particle is given by. For frequency f the quantum has energy hf where h is the constant introduced into the formula above now known as Plancks constant. Classical limit energy conservation etc but it is not derived from those arguments.
Schrodinger Equation The Schrodinger equation plays the role of Newtons laws and conservation of energy in classical mechanics - ie it predicts the future behavior of a dynamic system.
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ perg.phys.ksu.edu
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ www.researchgate.net
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ slideplayer.com
Source Image @ www.pinterest.com
Source Image @ www.physicsread.com
Source Image @ www.physicsread.com
If you are looking for Energy Formula Quantum Mechanics you've arrived at the right place. We ve got 10 images about energy formula quantum mechanics adding pictures, pictures, photos, wallpapers, and more. In these webpage, we additionally have number of images out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.
If the publishing of this internet site is beneficial to your suport by revealing article posts of this site to social media accounts which you have such as Facebook, Instagram among others or can also bookmark this blog page while using title What Is A Hamiltonian Operator Make use of Ctrl + D for laptop or computer devices with Glass windows operating system or Command + D for pc devices with operating-system from Apple. If you use a smartphone, you can also utilize the drawer menu of the browser you use. Be it a Windows, Macintosh, iOs or Android os operating-system, you'll be able to download images using the download button.
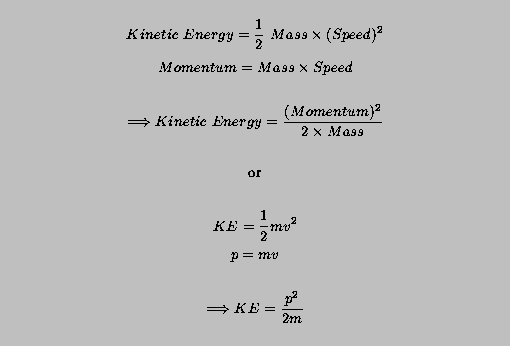



0 comments:
Post a Comment