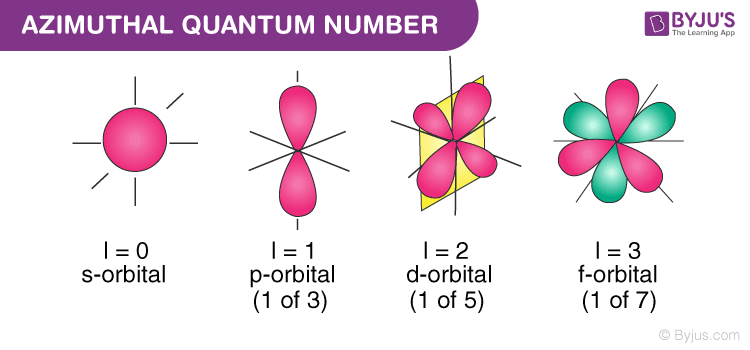
Azimuthal quantum number formula ~ And Azimuthal quantum numbers tells about shape of orbital. S p d and f each having a unique shape. Indeed recently is being searched by users around us, maybe one of you personally. Individuals now are accustomed to using the net in gadgets to see image and video information for inspiration, and according to the name of this article I will talk about about Azimuthal Quantum Number Formula With the help of the value of azimuthal quantum number we can determine the total number of energy sub-levels in a given energy level.
Source Image @ study.com
Angular Momentum Quantum Number Definition Example Video Lesson Transcript Study Com

Permitted values of l for a given value of n have 0 to n-1. Spin can either be 12 or -12. Your Azimuthal quantum number formula photos are ready in this website. Azimuthal quantum number formula are a topic that is being searched for and liked by netizens today. You can Download or bookmark the Azimuthal quantum number formula files here
Azimuthal quantum number formula - The Number Of Nodal Planes formula is defined as the number of planes having zero probability of finding an electron is calculated using no_nodes Azimuthal Quantum NumberTo calculate Number of Nodal Planes you need Azimuthal Quantum Number lWith our tool you need to enter the respective value for Azimuthal Quantum Number and hit the calculate button. M 2 l 1 values of l 1 to -1 including zero. This question does not show any research effort. Azimuthal Quantum Number s a quantum number for an.
How to find azimuthal quantum number. What is Azimuthal Quantum Number. Azimuthal Quantum Number The azimuthal quantum number is denoted by the symbol l. That is denoted by the symbol l and its value is same to the total number of angular nodes in the orbitalFor example if n 3 the azimuthal quantum number have the right to take.
From the azimuthal equation of the hydrogen Schrodinger equation comes a quantum number with the constraint. The azimuthal quantum number is the quantum number that describes the angular momentum of an electron in an atom. Azimuthal quantum number has been used from the time of Bohrs atomic model and was realized by Arnold Sommerfeld. Furthermore this quantum number determines the shapes of an orbital in.
The Pauli exclusion principle Wolfgang Pauli Nobel Prize 1945 states thatno two electrons in the same atom can have identical values for all four of their quantum numbers. It also designates the shape of. We hope this article on Quantum Number has helped you. For d-orbital ℓ 2.
It is unclear or not useful. Azimuthal Quantum Number Orbital Angular momentum Quantum Number The azimuthal or orbital angular momentum quantum number describes the shape of a offered orbital. Azimuthal quantum number l 1. But the total number of different values of l equal to n.
What are the orbitals present in the fourth principal energy level. Values of are from zero to n-1. Show activity on this post. The Orbital Angular Momentum of an object about a chosen origin is defined as the angular momentum of the centre of mass about the origin and is represented as L sqrt l l 1 h 2 pi or angular_momentum sqrt Azimuthal Quantum Number Azimuthal Quantum Number 1 Plancks Constant 2 pi.
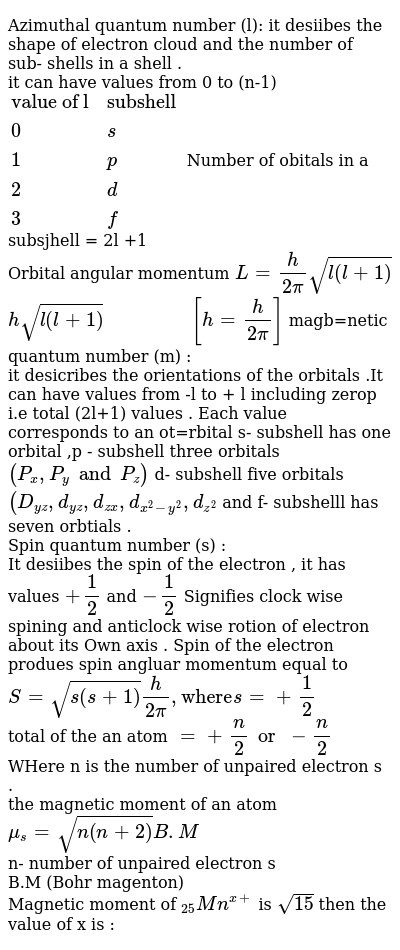
We know that l 1 is dum bell shaped called p orbital. It describes orientation of orbital in space under magnetic field which obtained due to angular momentum of electron and thus it relates to the value of l. Magnetic quantum number m. The other names of azimuthal quantum numbers are second quantum number orbital quantum number or orbital angular quantum number.
The Number Of Angular Nodes formula is defined as the number of points around the nucleus of an atom where the probability of finding an electron is zero and is represented as N n l or no_nodes Azimuthal Quantum Number. Principal quantum number n 3. M s ½ or -½. An electron can spin in only one of two directions sometimes called up and down.
The letter l denotes azimuthal quantum number. For f-orbital ℓ 3. The azimuthal quantum number is calculated by rml n-1 The magnetic quantum number is calculated by rmm -l to rml The spin quantum number has two values only 12 and 12 Learn About Principal Quantum. For s-orbital ℓ 0.
The value of ℓ tells the specific subshell. The representation of this azimuthal quantum number is l and is pronounced as ell. Therefore the magnetic quantum numbers l 0 1 2 3n 1. Spin Quantum Number ms.
The label azimuthal quantum number is also called as angular momentum quantum number because the values of l also govern the orbital angular momentum of the electron in a particular quantum mechanical state. Therefore we can also call it the orbital angular momentum quantum number. Azimuthal quantum number ℓ and magnetic quantum number m are defined when we do derivation for L 2 f ℓ ℓ 1 ℏ 2 f and L z f ℓ ℏ f. Azimuthal quantum number describes the shape of orbital.
Calculate the minimum and maximum number of electrons which have magnetic quantum number m 1 and spin quantum number s 12 in chromium. If you look at the standing waves in section 6432 the value of l can be related to the number of nodes and so the first standing wave n1 has l0 there is no node. Therefore the general geometric shapes of an electron cloud or orbitals define by the azimuthal or angular momentum quantum number. Magnetic quantum number ml 2 1 0 1 2.
Azimuthal Quantum Number s a quantum number for an atomic orbital that determines its orbital angular momentum. The second quantum number is often called the azimuthal quantum number l. So it is 2 electrons. Azimuthal Quantum Number denoted by ℓ Also known as orbitalangular momentum quantum number it refers to the subshell to which an electron belongs.
The value of l describes the shape of the region of space occupied by the electron. Specifies the orientation of the spin axis of an electron. Hence Designation for orbital with n 2 l 1 is 2p orbital. Azimuthal quantum number l 2.
It is denoted by. Which orbital designation has the highest energy. For p-orbita ℓ 1. And can have value n1 n2 n30.
While the azimuthal dependence of the wavefunction only requires the quantum number to be an integer the coupling to the colatitude equation further constrains that integer to be less than or equal to the orbital quantum number.
Source Image @ www.pinterest.com
Source Image @ www.priyamstudycentre.com
Source Image @ byjus.com
Source Image @ byjus.com
Source Image @ www.toppr.com
Source Image @ www.doubtnut.com
Source Image @ www.youtube.com
Source Image @ www.youtube.com
Source Image @ study.com
If you re looking for Azimuthal Quantum Number Formula you've come to the right place. We have 10 graphics about azimuthal quantum number formula adding pictures, pictures, photos, wallpapers, and much more. In such webpage, we also provide variety of graphics out there. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.
If the posting of this website is beneficial to your suport by expressing article posts of this site to social media marketing accounts that you have got such as for example Facebook, Instagram among others or may also bookmark this website page while using title Angular Momentum Quantum Number Definition Example Video Lesson Transcript Study Com Employ Ctrl + D for computer system devices with Windows operating-system or Command word + D for laptop devices with operating system from Apple. If you use a smartphone, you can even utilize the drawer menu in the browser you use. Be it a Windows, Mac pc, iOs or Android operating-system, you'll be in a position to download images using the download button.






0 comments:
Post a Comment