Formula for energy quantum of light ~ Energy emitted by the light bulb every second 100 J Energy of each quantum 331x10 -19 J Therefore number emitted per second 100331x10 -19 30x10 20 photons per second. λ wavelength of the light. Indeed lately is being hunted by users around us, perhaps one of you. Individuals are now accustomed to using the internet in gadgets to see video and image information for inspiration, and according to the title of the post I will discuss about Formula For Energy Quantum Of Light Photons carry momentum and energy from the source of light by which they are emitted.
Source Image @ study.com
Energy Momentum Of A Photon Equation Calculations Video Lesson Transcript Study Com

For light quanta we use the formula the energy of a photon plancks constant x frequency. E h f where. Your Formula for energy quantum of light image are available in this site. Formula for energy quantum of light are a topic that has been hunted for and liked by netizens now. You can Get or bookmark the Formula for energy quantum of light files here
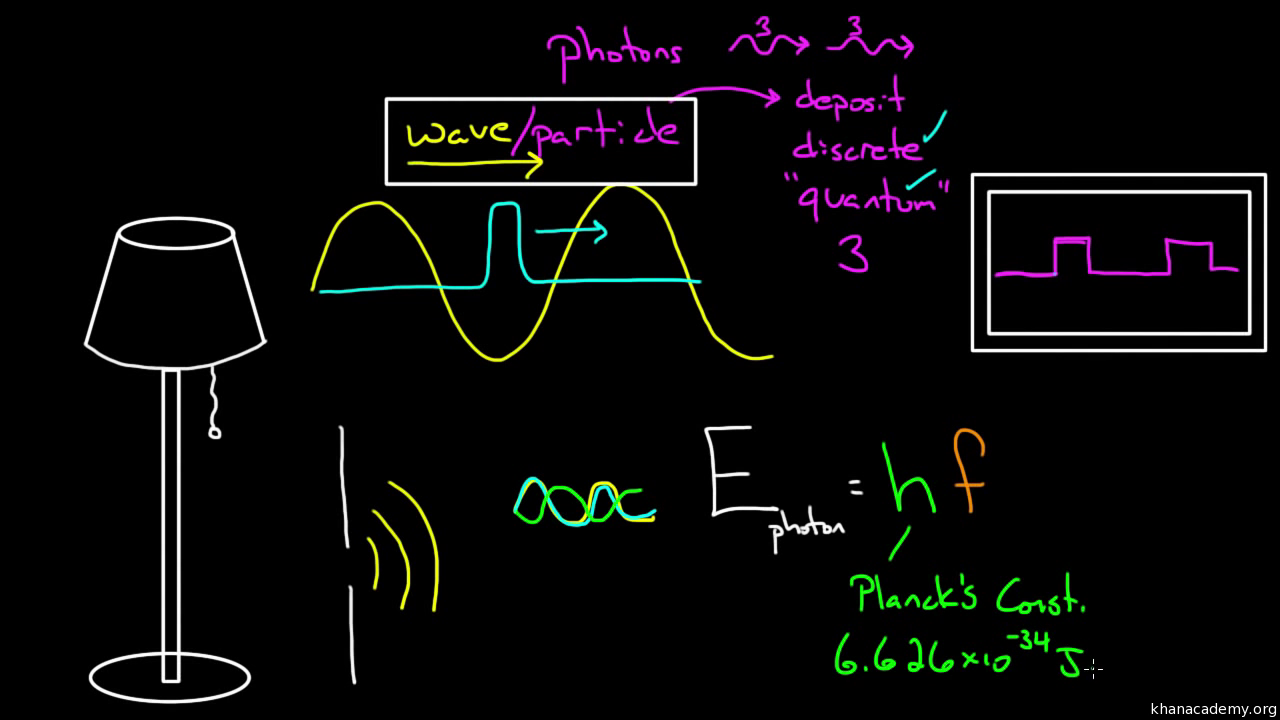
Formula for energy quantum of light - The is not consistent when the light is pictured as a wave. We call them photons and they carry the momentum and energy from our source of light. In this specific context a photon is a discrete bundle or quantum of electromagnetic or light energy. Because this is the smallest discrete amount of energy there can only be whole number quantities of photons.
Einstein and His Equation of the Photoelectric Effect. E Energy of the photon in Joules h Constant actually known as Plancks constant a really ugly number. F frequency of the light in units of per seconds 1seconds The second version of the formula is found by using the light properties formula c f and substituting that in for the above relation. This occurs at the vacuum speed of light more commonly just called the speed of light the formula for which is.
A photon is characterized either by wavelength λ or an equivalent energy E. A slightly different way would be to use Eλ hc with the wavelength in meters and solve for E then multiply the answer times Avogadros Number. The photon energy at 1 Hz is equal to 662607015 10 34 J. λ is the wavelength.
λ is the photons wavelength metres. Energy of Light 6626 10-31 c wm Where e Energy c Speed Of Light300000000 ms w Wavelength. It is also clear that there is a minimum light frequency for a given metal nu_o that for which the quantum of energy is equal to Phi Equation refEq1. C is the speed of light in vacuum - 299792458 metres per second.
If this quantum of energy absorbed exceeds the minimum energy needed for the electron to escape from the metal surface work function the electron is emitted with maximum KE. Where h is the Plancks constant and ν is the frequency of light. Therefore light must be made up of these quantas or packets of energy or quantum energy. E 663 1034 300 108 490 109 xJ.
More tightly bound electrons will emerge with KE less than the maximum value. Existing in a vacuum and constantly in motion photons have a constant speed of light to all observers. Scalar waves are standing waves that flash on and off This process creates energy patterns that are processed by our consciousness and DNA to create our external reality. According to both Planck and Einstein the energy of light is proportional to its frequency rather than.
H is the Planck constant - 662607015 10 34 m 2 kgs 1. That is equal to 4135667697 10 15 eV electronvolts. Where E is photon energy Joules. On the basis of Plancks quantum theory Einstein derived an equation for the photoelectric effect known as Einstein photoelectric equation.
In the photoelectric effect each electron absorbs a quantum of energy of radiation. E photon energy h Plancks constant 6626 10 34 Js c speed of the light and. Light below that frequency no matter how bright will not eject electrons. Also the electron can jump to a lower energy level by releasing a photon.
X 3614 x 1019Jphoton 6022 x 1023photon mol1 21763508 Jmol. Light quanta called photons are the smallest discrete amounts of light. Light consists of photons or quanta of energy energy in each photon is hν. E hc λ.
Therefore as the excitation of the hydrogen atom increases the minimum energy needed to free the electron diminishes. Again to excite the electron to the second excited state the energy required is E3-E1 -151 eV--136eV 1209 eV. As shown by the Compton effect light can behave like waves or particles. According to the Einstein-Plank relation we have E hν1.
That constant is called Plancks Constant and is given the symbol h. One electron volt equals the energy gained by an electron when its electric potential is changed by one volt. The energy constant for light is 6626 X 10-34 Josc meaning the energy of a single oscillation of light is constant irrespective of the lights frequency or wavelength. The above equation is.
The electron volt is the work done in moving 1 electron through a potential difference of. Therefore light is made up of packets of energy or quantum of energy. H 663 10 -34 J s Plancks Constant. E 400 1019xJ.
The energy of a photon is inversely proportional to the wavelength of a photon. A consistent explanation would be when the picture of light comes in discrete packages known as photons and every photon should have sufficient energy to eject a single electron. C is the speed of light 300 108xms. Energy is often measured in.
The Photon energy formula is given by Where. The on and off energy pattern is very simple but yet it has infinite potential. A more convenient energy unit in this regime is the electron volt eV. Dividing the answer by 1000 to make the change to kilojoules we get 2176 kJmol.
The result is how the energy depends upon the. C 2998 x 108 ms. If 1 Coulomb of charge is moved through a potential difference of 1 Volt then 1 Joule of work is done. The energy of a photon of visible light is very small being on the order of 4 10 19 joule.
This is the mysterious and amazing power of the intelligence of Creation. 1 eV 16 10 19 joule. According to Einstein-Planck relation E. This tells us that the kinetic energy is equal to the frequency of the light times a constant ie the slope of the line.
These particles consist of higher energy which is also called the quantum of radiation.
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ byjus.com
Source Image @ abyss.uoregon.edu
Source Image @ slideplayer.com
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ www.tectechnicsclassroom.tectechnics.com
Source Image @ byjus.com
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ www.khanacademy.org
If you are looking for Formula For Energy Quantum Of Light you've come to the right place. We ve got 10 graphics about formula for energy quantum of light including images, photos, photographs, wallpapers, and more. In such web page, we also have number of graphics available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
If the publishing of this web page is beneficial to your suport by sharing article posts of this site to social media accounts you have such as for example Facebook, Instagram and others or can also bookmark this blog page with the title Photon Energy Video Photons Khan Academy Employ Ctrl + D for laptop devices with Home windows operating-system or Command + D for personal computer devices with operating-system from Apple. If you use a smartphone, you can even use the drawer menu of this browser you use. Be it a Windows, Macintosh, iOs or Android operating system, you'll still be in a position to download images utilizing the download button.



0 comments:
Post a Comment