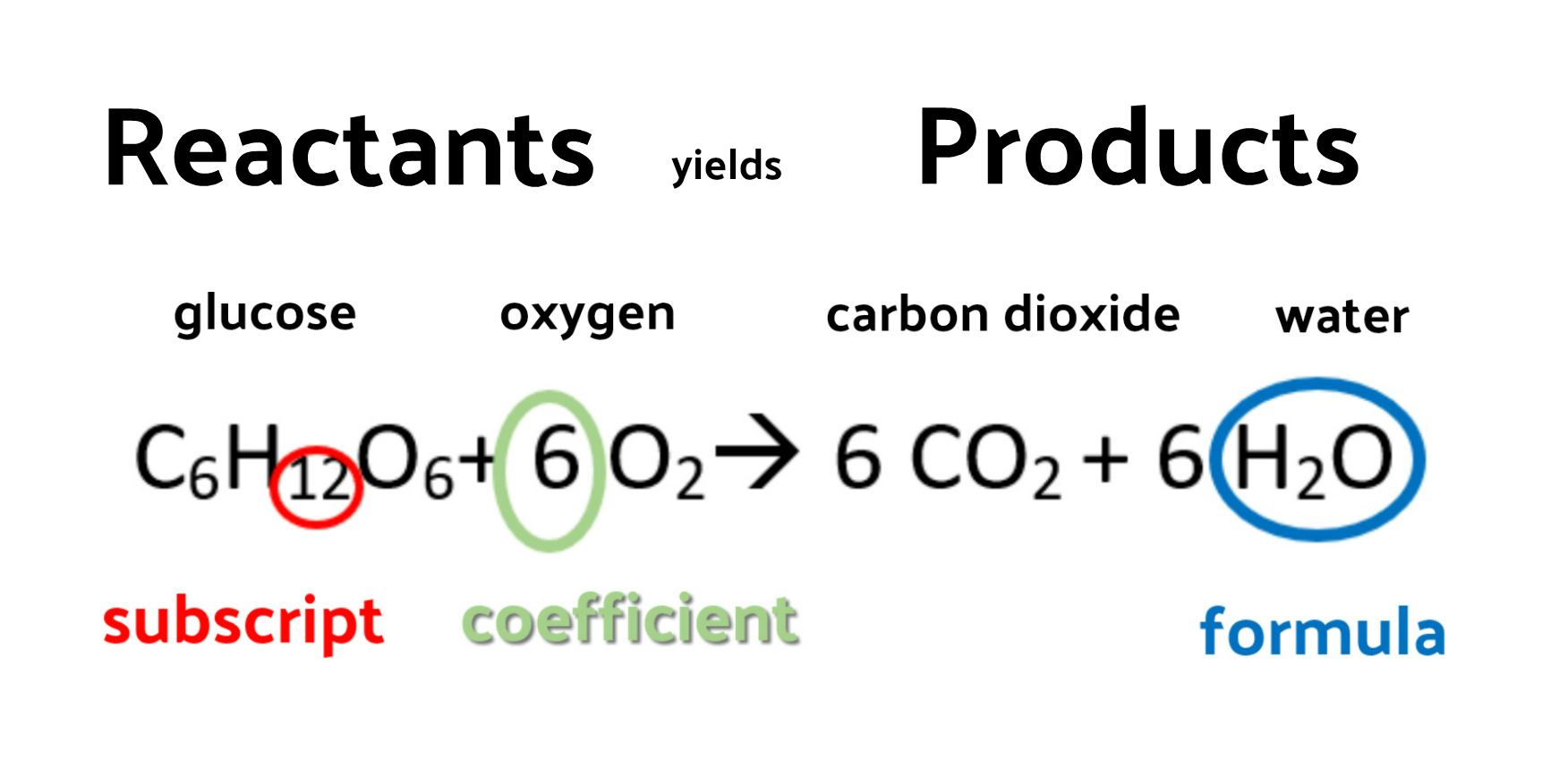
Science formulas reactions ~ The LHS consists of the reactants and the RHS consists of the products. Chemical equations model the changes that happen in chemical reactions. Indeed lately has been searched by consumers around us, perhaps one of you personally. Individuals now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about Science Formulas Reactions Take a look at this word equation for the reaction.
Source Image @ onlinesciencenotes.com
Chemical Reactions Balanced And Unbalanced Chemical Equations Online Science Notes

For example a search on Mg will find reactions with Mg and Mg 2. Both oxidation and reduction take place simultaneously. Your Science formulas reactions image are ready. Science formulas reactions are a topic that has been hunted for and liked by netizens now. You can Get or bookmark the Science formulas reactions files here
Science formulas reactions - CuO H 2 Cu H 2 O. Provided below is a list of the chemical formulas of some common chemical compounds along with their molecular weights. The list of chemical reaction available here is from Class 10th Science NCERT tex. The reaction of the formation reaction of sulphuric acids is given below.
Any reaction in which two or more substances combine to form a single product is a direct union or combination reaction. How the Reaction Works. A chemical equation is the representation of the chemical reactions. The half-life formula for various reactions is given below.
The mathematical expression can be employed to determine the half-life for a zero-order reaction is t 12 R 0 2k. The reaction between baking soda and vinegar actually occurs in two steps but the overall process can be summarized by the following word equation. Chemical Reaction and equation is a part of science which come under chemistry our expert uploaded all required notes for Chemical Reaction and equation in few pages. Types of Chemical Reactions Type of reaction Generalized formula Specific Example Combustion HC O 2 H 2 O CO 2 2C 2 H 6 7O 2 6H 2 O 4CO 2 Synthesis A B AB 2Na Cl 2 2NaCl Decomposition AB A B 2H 2 O 2H 2 O 2 Single Replacement A.
A chemical reaction is described by a chemical equation an expression that gives the identities and quantities of the substances involved in a reaction. This is possible only when you have the best CBSE Class 10 Science study material and a smart preparation plan. In a neutralization or acidbase reaction the net ionic equation will usually be. Enter the name formula or CAS registry number of the core ion as a reactant.
The informal use of the term formula in science refers to the general construction of the relationship between given quantities. Formulas are utilized in physics to precise relationships between different quantities like temperature mass or charge. It is essential to note that the half-life formula of a reaction varies with the reactions order. C O 2 CO 2 g heat.
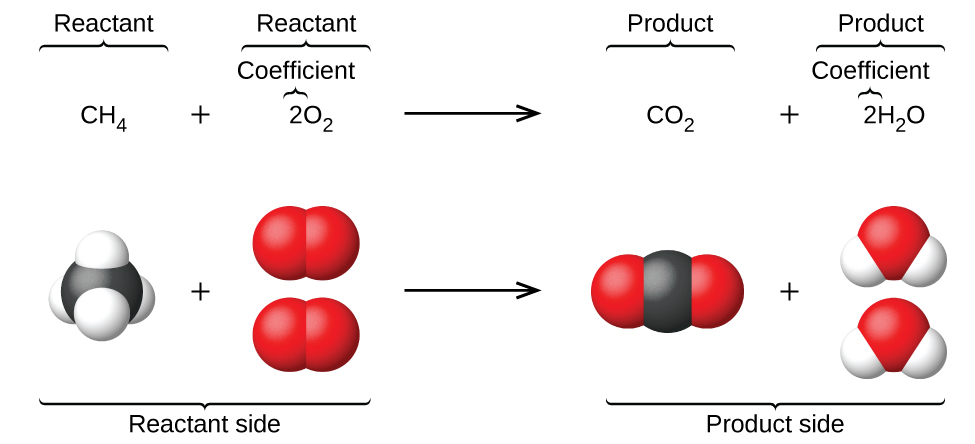
Chemical equations are typically written using the chemical formulas for the reactants and products. Balancing chemical equation is the process of equalising the number of each. You can think about it this way. For example the reaction of mercury with oxygen to produce mercuric oxide would be expressed by the equation.
This pdf sheet of chapter Chemical Reaction and equation is useful for last minutes revision before exam. Please note that if you enter the neutral formula for the core ion you will get reactions for all ions with the given formula. Reaction in which heat is evolved. C s O 2 g CO 2 g Heat.
ZnCO 3 Heat ZnO CO 2. Balance the given chemical reaction as per the instructions below. Check chapter-wise important chemical reactions of CBSE Class 10th Science. Formulas used in science almost always require a selection of units.
H aq OH aq H 2 Ol There are a few acidbase reactions that produce a precipitate in addition to the water molecule shown above. The purpose of a chemical equation is to express this relation in terms of the formulas of the actual reactants and products that define a particular chemical change. CeNaOH H2SO4 - Na2SO4 H2O Write the number of atoms of various elements in the reactants and products. Copper oxygen copper oxide copper and oxygen are the reactants because they are on the left of the arrow.
A chemical reaction in which heat energy is absorbed. The general form of a direct union reaction is A B AB This type of reaction generally takes place between the following types of compounds. Chemical equations are used to show chemical reactions. A chemical equation shows the starting compoundsthe reactantson the left.
For the first-order reaction the half-life is defined as t 12 0. Candidates who are ambitious to qualify the Class 10 with good score can check this article for Notes. This equation is easily balanced by placing the coefficient 2 in front of molecule HCl to form the balanced equation Mg 2 HCl MgCl2 H2. In a chemical reaction one or more reactants are transformed into products.
Chemical formulae provide a way to represent any chemical substance using the symbol of the elements present in it. Class 10 Science Chemical Reactions and Equations Get here the Notes for Class 10 Science Chemical Reactions and Equations. Baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion. 2Sl 3O2gH2Ol 2H2SO4aq 2 S l 3 O 2 g H 2 O l 2 H 2 S O 4 a q To learn more on the list of chemical reactions Register with BYJUS and download our app.
A metal non-metal 2 Na Cl2 2 NaCl sodium chloride Fe S FeS. Reaction involving both oxidation and reduction simultaneously CuO s H 2 Cu s H 2 O l. Introducing chemical reactions Chemists use symbols and formulae to represent elements ions and compounds. If you required detail science class 10th than visit class 10 science sectionAcademic team of.
A chemical reaction in which heat energy is evolved. 1 atom Mg 2 compounds HCl combines in a reaction to form the products of 1.
Source Image @ onlinesciencenotes.com
Source Image @ chem.libretexts.org
Source Image @ www.pinterest.com
Source Image @ www.mikeblaber.org
Source Image @ www.pngwing.com
Source Image @ byjus.com
Source Image @ ncerthelp.com
Source Image @ www.researchgate.net
Source Image @ www.youtube.com
If you re searching for Science Formulas Reactions you've arrived at the perfect location. We ve got 10 images about science formulas reactions including images, photos, pictures, backgrounds, and much more. In such web page, we also provide variety of images available. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.
If the publishing of this webpage is beneficial to your suport by sharing article posts of the site to social media marketing accounts that you have got such as for example Facebook, Instagram and others or may also bookmark this blog page along with the title Chemical Reactions 6 Of 11 Quick Review 5 Types Of Chemical Reactions Youtube Use Ctrl + D for laptop devices with Glass windows operating system or Command + D for laptop devices with operating system from Apple. If you are using a smartphone, you can also utilize the drawer menu on the browser you utilize. Be it a Windows, Macintosh, iOs or Android os operating system, you'll still be able to download images utilizing the download button.



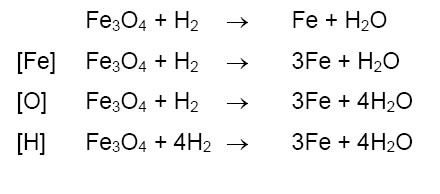


0 comments:
Post a Comment