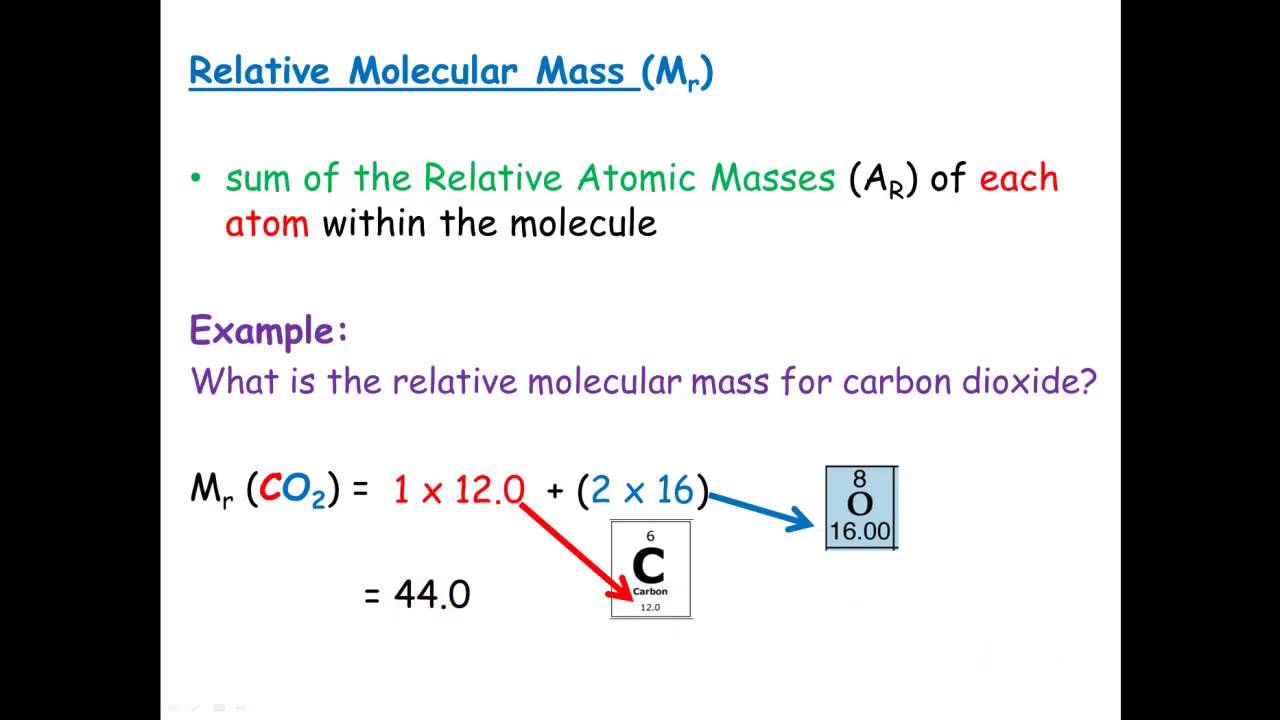
Cognito science relative formula mass ~ Relative formula mass Q3. H 2 N 2 NH 3 7 marks. Indeed lately is being searched by consumers around us, perhaps one of you. Individuals now are accustomed to using the internet in gadgets to see video and image data for inspiration, and according to the name of the post I will discuss about Cognito Science Relative Formula Mass For example carbon dioxide has the formula CO₂ so the relative formula mass is 12 16 2 44.
Source Image @ www.youtube.com
Gcse Science Revision Chemistry Relative Formula Mass Youtube

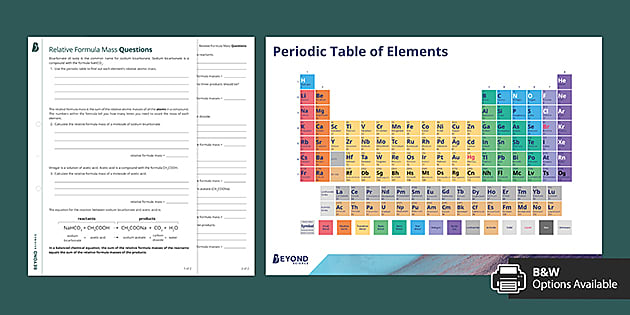
The symbol for the relative formula mass is M r and it refers to the total mass of the molecule. Beyonds Relative Formula Mass Worksheet helps students to understand this core concept as part of Topic 1 in Chemistry for Edexcel GCSE ScienceOffering a simple explanation of relative formula mass our Relative Formula Mass Worksheet provides a series of questions to reinforce learning as well as a periodic table of elements for referenceSample questions from our Relative Formula Mass. Your Cognito science relative formula mass image are available. Cognito science relative formula mass are a topic that has been hunted for and liked by netizens now. You can Get or bookmark the Cognito science relative formula mass files here
Cognito science relative formula mass - Define what is meant by the relative formula mass of a compound. The relative formula mass of a substance made up of molecules. The symbol for the relative formula mass is M r and it refers to the total mass of the molecule To calculate the M r of a substance you have to add up the relative atomic. The result should be a whole number or very close to a whole number.
This simply means the calculation is performed using relative atomic weight values for the elements which are based on the natural isotopic ratio of elements found in Earths atmosphere and crust. The relative formula mass of a compound is the sum of the relative atomic masses of the atoms in the numbers shown in the formula. Textgmol nonumber Divide the molar mass of the compound by the empirical formula mass. The first step in determining the molecular formula of a compound is to calculate the empirical mass from its empirical formula.
Answer sheet now uploaded please notify me of any errors. Relative formula Mass worksheet and answer sheet. This is calculated from the mass number and relative abundances of all the isotopes of a particular element. 3 atomic mass of calcium 2 atomic mass of phosphorus 8 atomic mass of oxygen 310.
The formula unit mass of Ca 3 PO 4 2 is Answer. A related term you should know is relative formula mass relative formula weight. Ranges from easy to very hard. Calculating relative formula mass.
The relative formula mass of a substance is the sum of the relative atomic masses of the elements present in a formula unit. Is the sum of the relative atomic masses of the atoms in the numbers shown in the formula. The relative formula mass of a compound is calculated by adding together the relative atomic mass values for all the atoms in its formula. Links are made but may not be fully articulated and or precise.
Calculate the percentage by mass of potassium in potassium nitrate. Relative Molecular Mass of a molecule is calculated by adding together the relative atomic masses of the atoms in the chemical formulae. MgCl2 has a relative formula mass of 95 since there is one Magnesium atomic mass 24 and two chloines each with atomic mass of 355. We then look at how to calculate relative formula mass for a compound.
The empirical formula mass for this compound is therefore 8113 amuformula unit or 8113 gmol formula unit. A homework sheet with 20 RMM to work out it does require a periodic table. Relative formula mass has the symbol M r. Balance the equation then fill in the relative formula mass in the boxes.
2 marks 1 a i Potassium nitrate is a fertilser with the formula KNO3 The relative formula mass Mr of potassium nitrate is 101. Let me know if there is a typo please. To calculate the M r of a substance you have to add up the relative atomic masses of all the atoms present in the formula. TextEmpirical formula molar mass EFM 1384.
Relative formula mass M. Sum the masses to determine the molar mass represented by the formula. If the substance is made of simple molecules this mass may also be called the relative molecular mass. The symbol for the relative atomic mass is A r.
Relative Formula Mass Definition. The symbol for relative formula mass is M r. Conservation of mass and balanced symbol equations Relative formula mass Mass changes when a reactant or product is a gas Moles Using moles to balance equations Limiting reactants Concentration of solutions Percentage yield Atom economy Volumes of gases All of these lesson presentations and accompanying resources are. The definition of Relative Molecular Mass Mr also referred to as Molar Mass is The mass of a single molecule on a scale on which the mass of an atom of carbon 12 has a mass of 12 atomic mass units.
4057 g nicotine 02500 mol nicotine 1623 g mol 4057 g nicotine 02500 mol nicotine 1623 g mol. Name Formula Relative formula mass Boiling point in C methane CH 4 16 160 ethane C 2 H 6 30 90 propane C 3 H 8 44 40 butane C 4 H 10 58 1 pentane C 5 H 12 72 hexane C 6 H. The relative formula mass is JUST the relative atomic mass of all of the atoms in the molecule added up. First we learn what is meant by relative formula mass.
Relative formula mass. R of aspirin 180 4 marks Number of moles 0 4. To do this look up the mass of each element present in the compound and then multiply that number by the subscript that appears after its symbol in the formula. Calculate the molar mass for nicotine from the given mass and molar amount of compound.
What is relative formula mass and relative molecular mass. Moles are units used to measure substance amount. Calculate the empirical formula mass EFM. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams.
The topics covered within these lessons include. Fractextmolar masstextEFM frac277 gmol1384 gmol 2 nonumber. Relative atomic masses Ar. Using the periodic table state the relative formula mass of the following compounds.
A MgO 2 marks b NH 3 2 marks Q5. One molecule of water H 2 O would weigh 1802 amu 2100797 amu for H 159994 amu for O and a mole of water molecules would weigh 1802 grams. In this GCSE Chemistry video we look at relative formula mass.
Source Image @ www.twinkl.co.uk
Source Image @ www.youtube.com
Source Image @ www.youtube.com
Source Image @ perangkatsekolah.net
Source Image @ www.youtube.com
Source Image @ www.slideserve.com
Source Image @ www.youtube.com
Source Image @ www.tes.com
Source Image @ www.youtube.com
If you are looking for Cognito Science Relative Formula Mass you've come to the perfect location. We ve got 10 images about cognito science relative formula mass adding images, photos, photographs, wallpapers, and much more. In these page, we also provide variety of images out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.
If the publishing of this website is beneficial to our suport by revealing article posts of the site to social media marketing accounts that you have such as Facebook, Instagram among others or may also bookmark this website page while using title Relative Atomic Mass Properties Of Matter Chemistry Fuseschool Youtube Use Ctrl + D for computer devices with Home windows operating-system or Command + D for laptop or computer devices with operating system from Apple. If you are using a smartphone, you can even use the drawer menu of this browser you use. Whether its a Windows, Macintosh personal computer, iOs or Android os operating system, you'll be able to download images using the download button.






0 comments:
Post a Comment