Formula orbital quantum number ~ The orbital quantum number arises when solving the Schrodingers equation. No of orbitals 22 4 orbitals 2s and three 2p orbitals 3. Indeed lately has been searched by users around us, maybe one of you personally. People now are accustomed to using the internet in gadgets to see image and video data for inspiration, and according to the title of the post I will discuss about Formula Orbital Quantum Number Principal Quantum Number n.
Source Image @ www.youtube.com
The Magnetic Quantum Number Ml Youtube

Spin can either be 12 or -12. The first three n n n ℓ ell ℓ and m ℓ m_ell m ℓ come from the solution to the spherical Schrödinger equation and describe the orbital of the electron. Your Formula orbital quantum number pictures are available in this site. Formula orbital quantum number are a topic that is being hunted for and liked by netizens now. You can Download or bookmark the Formula orbital quantum number files here
Formula orbital quantum number - The possible total number of orbitals in a given subshell for n 4 and ℓ 3 is 23 1 7. Principle quantum number is one of the most critical quantum numbers that tells about. The values of ℓ are the eigenvalues of the angular momentum operator squared and the spherical harmonics Y are its eigenfunctions. L 3 m 3 2 1 0-1 -2 -3 the orbital is f.
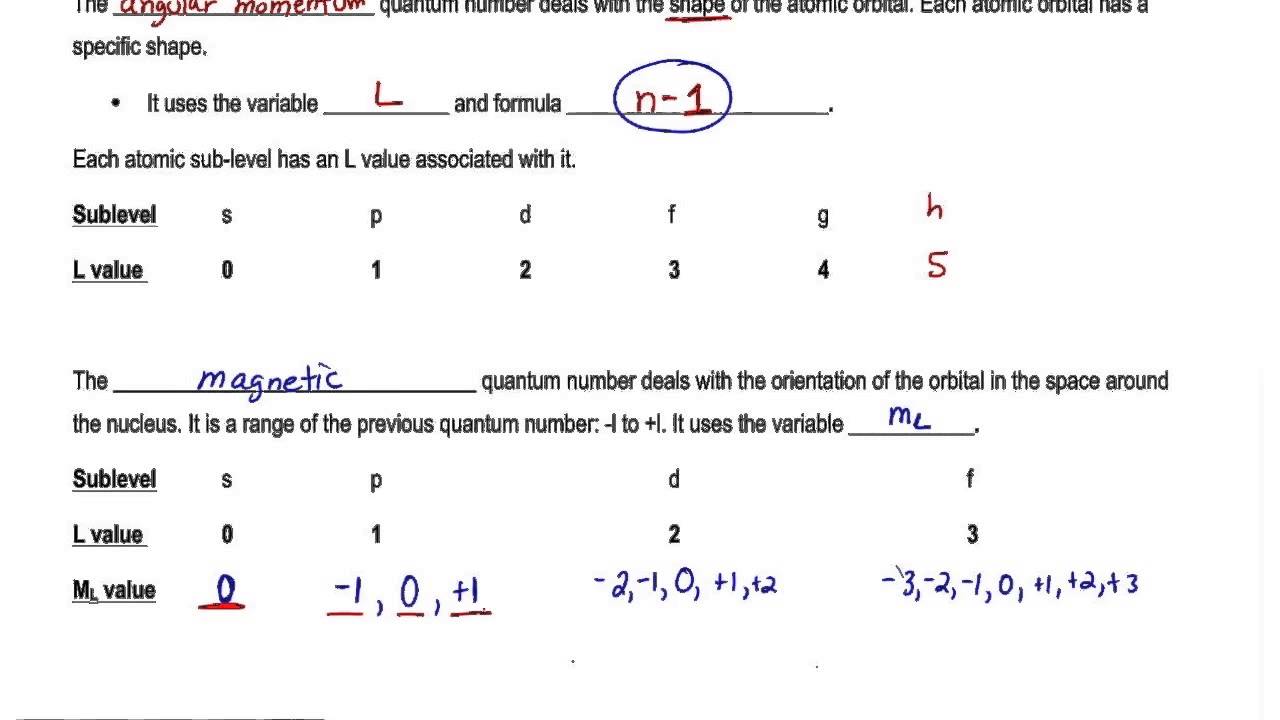
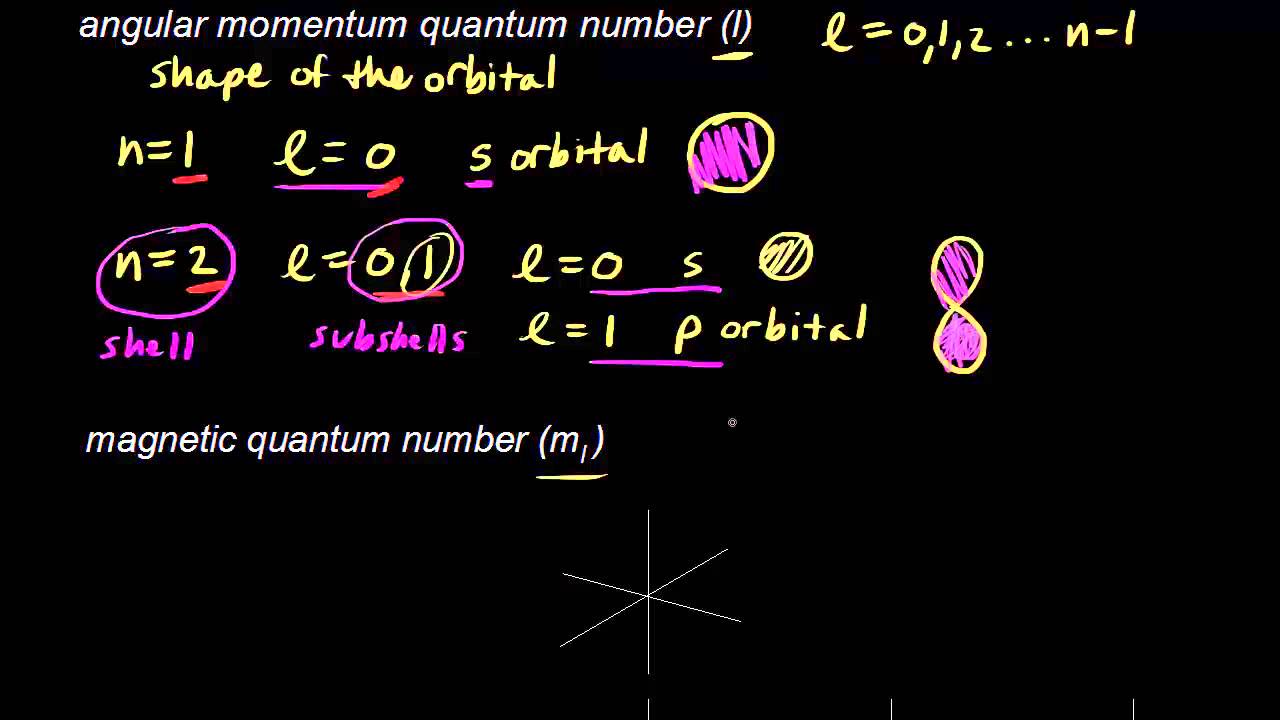
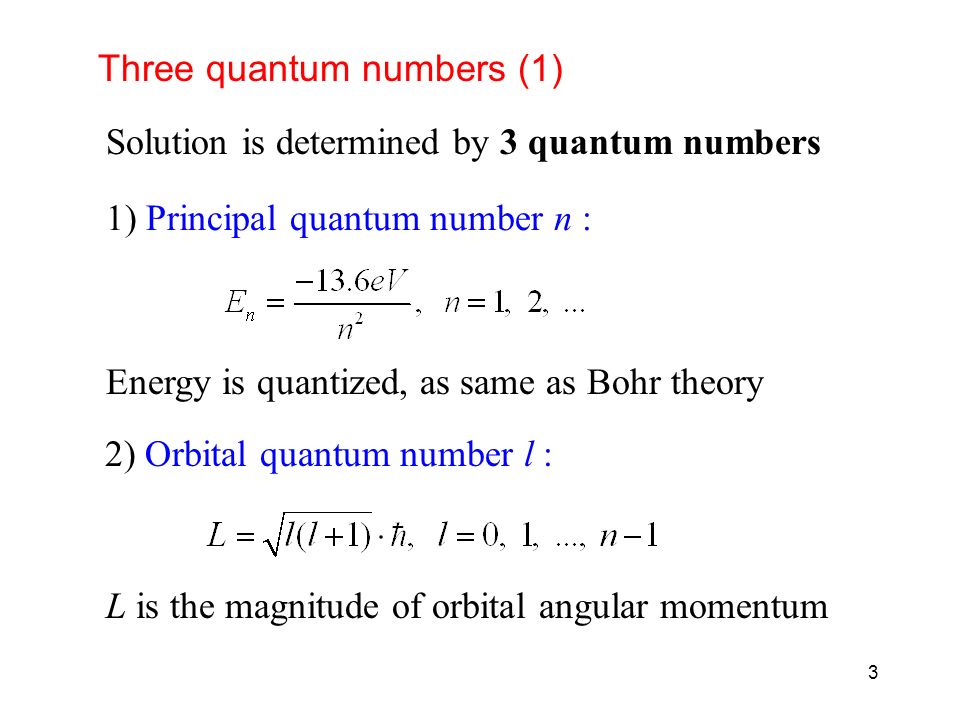
Circular path of electron around nucleus is called orbit and it shown by principal quantum number n. This magnetic moment is due to the orbital motion It may be calculated by the formula Orbital magnetic moment l l 1 h 2 π. Angular momentum quantum number l n 1. N 1 2 3 8.
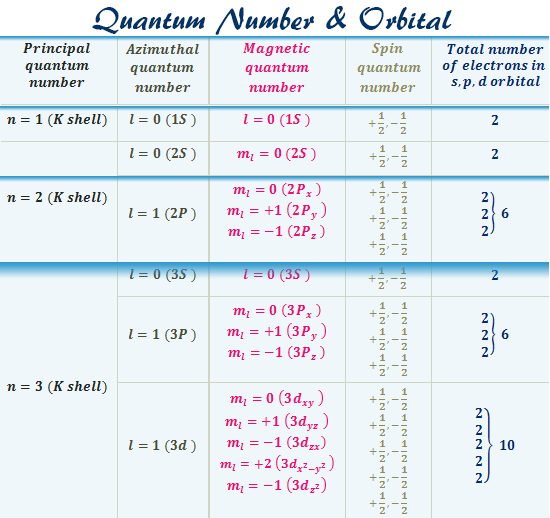
Magnetic quantum number ml 2 1 0 1 2. The number of orbitals in a subshell is therefore 2l 1. Numbers of possible orbitals when principle quantum number equal to four 1 4s 3 4p 5 4d 7 4f 16. The Total magnetic quantum number value formula is defined as the number which divides the subshell into individual orbitals which hold the electrons and is represented as m 2 l1 or magnetic_quantum_number 2 Azimuthal Quantum Number1.
No of orbitals 12 1 orbital 1s 2. The spatial area in which there is highest probability of finding an electron is called orbital and shown by angular quantum number l. Spin quantum number s. A kind of coordinate system.
Each electron in an atom is described by four different quantum numbers. The number of value of m determines the number of orbital of a particular type. Azimuthal Quantum Number s a quantum number for an atomic orbital that determines its orbital angular momentum. For s orbital l0 Orbital magnetic moment 0.
A set of the four quantum numbers describes the unique properties of one specific electron in an atom. Answer 1 of 3. For p orbital l1 Orbital magnetic moment l l. Since each set is unique they serve as a way of uniquely naming individual electrons ie.
For the 3d orbital Principal quantum number n 3. S p d and f are different types of orbital. The first three n l m l specify the particular orbital of interest and the fourth m s specifies how many electrons can occupy that orbital. Number of orbit is the energy level of electrons revolving in a particular orbit.
What is the formula of principal quantum number. Azimuthal quantum number l 2. 12 1 22 4 32 9. -3 -2 -1 0 1 2 3.
Each orbital can accommodate 2 electrons so there will be a total of 14 or 7 2 electrons in 3f subshell. Each l value represents a specific orbit named after the description of the hydrogen spectrum such s for sharp l 0 p for principal l 1 d for diffuse l 2 f for fundamental l 3 etc. The Total number of orbitals of principal quantum number is the maximum energy levels in an atom where electrons revolve around the nucleus and is represented as t n 2 or total_number_of_orbitals Number of orbit 2. Calculate the minimum and maximum number of electrons which have magnetic quantum number m 1 and spin quantum number s 12 in chromium.
No of orbitals in a shell of principle quantum number n is n2 for eg. L azimuthal quantum number of orbital. Electron has two types of velocity orbital velocity and axial velocity. How many possible numbers of orbitals of an atom when the principal quantum number equal to four.
There is one orbital in an ssubshell l 0 three orbitals in a psubshell l 1 and five orbitals in a dsubshell l 2. Orbital velocity is velocity obtained by movement of electrons around nucleus. The values of magnetic quantum numbers will be -3 -2 -1 0 1 2 and 3. The number of orbitals in a shell is the square of the principal quantum number.
29 rows These orbitals are designated as P x P y P z orbitals. L 2 Y ℓ m θ ϕ ℏ 2 ℓ ℓ 1 Y ℓ m θ ϕ The spherical. No of orbitals 32 9 orbitals 3s three 3p and five 3d orbitals 4.
Source Image @ www.meritnation.com
Source Image @ www.khanacademy.org
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ www.priyamstudycentre.com
Source Image @ www.youtube.com
Source Image @ slideplayer.com
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ study.com
Source Image @ study.com
If you re looking for Formula Orbital Quantum Number you've reached the right location. We ve got 10 graphics about formula orbital quantum number adding images, pictures, photos, wallpapers, and much more. In these webpage, we also have number of images available. Such as png, jpg, animated gifs, pic art, symbol, black and white, transparent, etc.
If the posting of this site is beneficial to your suport by spreading article posts of this site to social media accounts that you have got such as Facebook, Instagram and others or may also bookmark this blog page using the title Magnetic Quantum Number Definition Example Video Lesson Transcript Study Com Use Ctrl + D for pc devices with House windows operating system or Command line + D for computer devices with operating-system from Apple. If you use a smartphone, you can also utilize the drawer menu from the browser you utilize. Be it a Windows, Macintosh, iOs or Google android operating system, you'll be in a position to download images using the download button.



0 comments:
Post a Comment