Empirical formula for science ~ So your empirical formula is the ratio of the two. An empirical formula is one that shows the lowest whole-number ratio of the elements in a compound. Indeed lately is being searched by users around us, perhaps one of you personally. Individuals are now accustomed to using the internet in gadgets to see video and image information for inspiration, and according to the name of this article I will talk about about Empirical Formula For Science The molecular formula must be twice the empirical formula.
Source Image @ www.scienceinschool.org
Classic Chemistry Finding The Empirical Formula Science In School
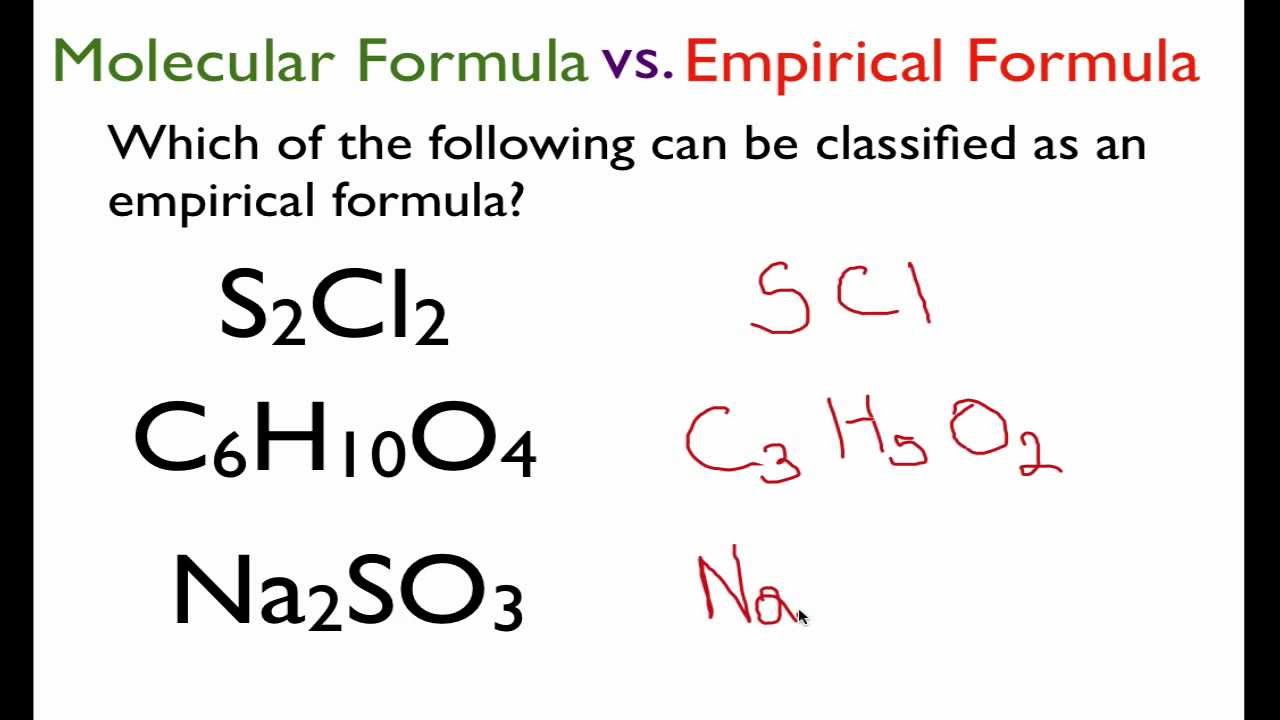
The empirical formula of a compound is the chemical formula which expresses the simplest whole number ratio of the atoms of the various elements present in one molecule of the compound. For example in hydrogen peroxide there is one part by mass of hydrogen for every 16 parts by mass of oxygen. Your Empirical formula for science pictures are available. Empirical formula for science are a topic that has been searched for and liked by netizens now. You can Find and Download or bookmark the Empirical formula for science files here
Empirical formula for science - Thus H 2 O is composed of two atoms of hydrogen and 1 atom of oxygen. Finally convert numbers to the whole numbers. The empirical formula of a compound. To write the empirical formula for an ionic compound we use the periodic table to identify the metallic cation and the nonmetallic anion and write their symbols and charges.
The atomic mass is given by B 3 H 1081 3 1 1381u. Molecular Formula n Empirical Formula is the general relationship between the empirical and molecular formulas. This set of whole numbers will be the subscripts in the empirical formula. The empirical formula of a compound gives the simplest ratio of the number of different atoms present whereas the molecular formula gives the actual number of each different atom present in a molecule.
The percent composition of a compound was found to be 63 5 silver 8 2 nitrogen and 28 3 oxygen. The simplest formula or the empirical formula provides the lowest whole number ratio of atoms existent in a compound. Elemental analysis is described as a process for the calculation of the empirical formula for a compound based on the percent composition of that compound. Is the simplest whole number ratio of atoms.
Compute the empirical formula of this compound. But you know that for every carbon theres a hydrogen. Empirical formula worksheet empirical formula a formula showing the smallest whole number mole ratio. Chlorine gas is passed over phosphorus it is found that 628g of phosphorus yield 1054g of a product.
If the formula is simplified then it is an empirical formula. The concept of empirical formula in the field of chemistry is very useful in finding out the elemental ratios of compounds that are similar in composition. A compound contains 8879 oxygen element and 1119 hydrogen element. We then determine the ratio of each element to ensure a total charge of zero.
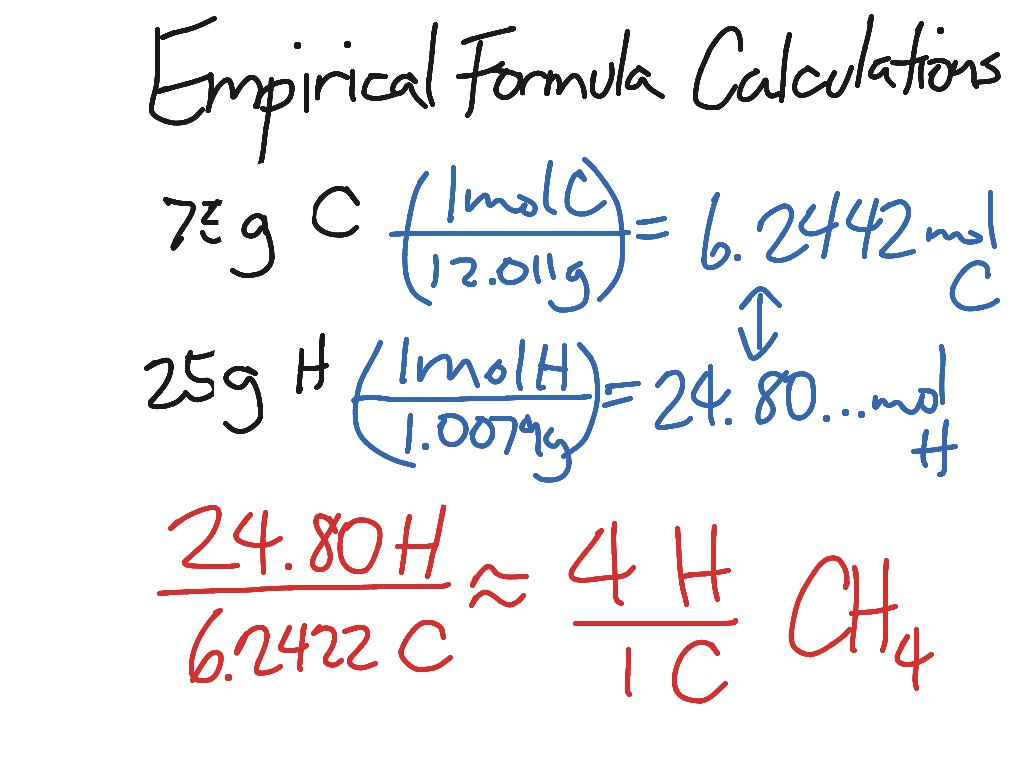
An empirical formula of a substance is found using the masses and relative atomic masses of the elements it contains. It can be used as a standard value when several ionic compounds are compared regarding their atomic structure chemical properties and percentage of elemental composition. But the measured molecular mass for Boron atom is given as 2766u. 1 a compound with an empirical formula of c2oh4 and a molar mass of 88 grams per mole.
The ratios hold true on the molar level as well. The molecular formula for hydrogen peroxide is H2O2. You dont know that each atom actually has six of these. Hey guys Im stuck on some work ive got to do for later this week heres the question.
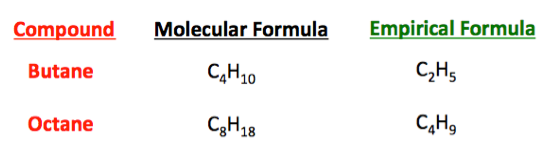
The empirical formula for a molecule is the lowest whole number ratio of atoms the molecular formula can be broken down into. Empirical formula C 6 H 11 NO. Steps for Determining an Empirical Formula. The way to go back you can go from the molecular formula to the empirical formula very easily.
Solved Examples on Empirical Formula. Empirical models are known to provide faster screw compressor selections and performance calculations since these are based on relatively simple mathematical relationships. The empirical formula is the simplest type of chemical formula as it shows the relative number of atoms of each element in a given compound. An empirical formula tells us the relative ratios of different atoms in a compound.
The empirical formula is a chemical formula that represents the simplest ratio of atoms in the chemical formula of the compound. For every hydrogen theres a carbon. Empirical models provide reasonably accurate performance predictions when the operating conditions of the compressor are closer to the test conditions in which the empirical model is based. The empirical formula is the simplest whole-number ratio of atoms in a compound.
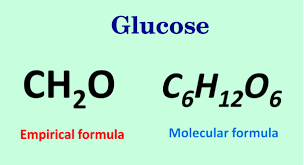
The empirical formula of benzene is CH hydrogen peroxide is HO Glucose is CH2O. The empirical formula for hydrogen peroxide is HO. The law of conservation of mass applies to closed and non-enclosed systems. Empirical formula show the simplest integer ratio of the atoms of the elements in a compound.
So i think ive got to work out the ratios to get the formul. A chemical formula showing the simplest ratio of elements in a compound rather than the total number of atoms in the molecule CH 2. Definition of empirical formula. Molecular formula show the actual number of atoms of the elements in a compound.
Work out the empirical formula of the product. Likewise 10 mole of H2O is composed of 20 moles of hydrogen and 10 mole of oxygen. R times whole number Empirical Formula. N molecular formulaempirical formula 2766 1381 2766 1381 2.
The relative number of atoms of every element in the compound is provided by this formula. Empirical formulas worksheet 1 directions. By using the expression Molecular formula n empirical formula. Putting value of n 2 in the empirical formula we get molecular formula as.
What is empirical and molecular formula. C3H72 or C6H14 Two kinds of data are needed to determine the molecular formula of a compound. 1 its composition from which we can calculate its empirical formula and 2 its molecular weight. For example consider sugar whose molecular formula is.
It is determined using data from experiments and therefore empirical.
Source Image @ twitter.com
Source Image @ www.youtube.com
Source Image @ study.com
Source Image @ sciencenotes.org
Source Image @ www.showme.com
Source Image @ chemcollective.org
Source Image @ thefactfactor.com
Source Image @ socratic.org
Source Image @ study.com
If you re searching for Empirical Formula For Science you've reached the right place. We ve got 10 images about empirical formula for science including pictures, pictures, photos, wallpapers, and more. In these web page, we additionally have number of graphics available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.
If the posting of this internet site is beneficial to your suport by posting article posts of this site to social media accounts which you have such as Facebook, Instagram among others or can also bookmark this blog page with the title What Is A Chemical Formula Definition Types Examples Video Lesson Transcript Study Com Make use of Ctrl + D for laptop or computer devices with Glass windows operating-system or Demand + D for laptop devices with operating system from Apple. If you use a smartphone, you can also use the drawer menu from the browser you utilize. Whether its a Windows, Mac pc, iOs or Android os operating system, you'll be in a position to download images utilizing the download button.





0 comments:
Post a Comment