Quantum number formulas ~ M s 12 -12. The fourth quantum number is independent of the first three allowing the first three quantum numbers of two electrons to be the same. Indeed lately has been hunted by users around us, perhaps one of you personally. Individuals now are accustomed to using the internet in gadgets to view video and image data for inspiration, and according to the title of this post I will discuss about Quantum Number Formulas Also certain macroscopic systems it directly applies to.
Source Image @ www.youtube.com
The Magnetic Quantum Number Ml Youtube

Angular momentum quantum number l n 1. Because angular momentum is a vector the Spin Quantum Number s has both a magnitude 12 and direction or -. Your Quantum number formulas photos are ready. Quantum number formulas are a topic that has been searched for and liked by netizens now. You can Download or bookmark the Quantum number formulas files here
Quantum number formulas - 63 Quantum Numbers In this section we write down the quantum numbers which characterize the solutions to Schrödingers equation. The magnetic quantum number can be derived from solving the azimuthal equation of the hydrogen Schrodinger equation. M l - ℓ to ℓ -101 Spin Quantum Number. Quantum mechanics takes into account the dual behavior of matter.
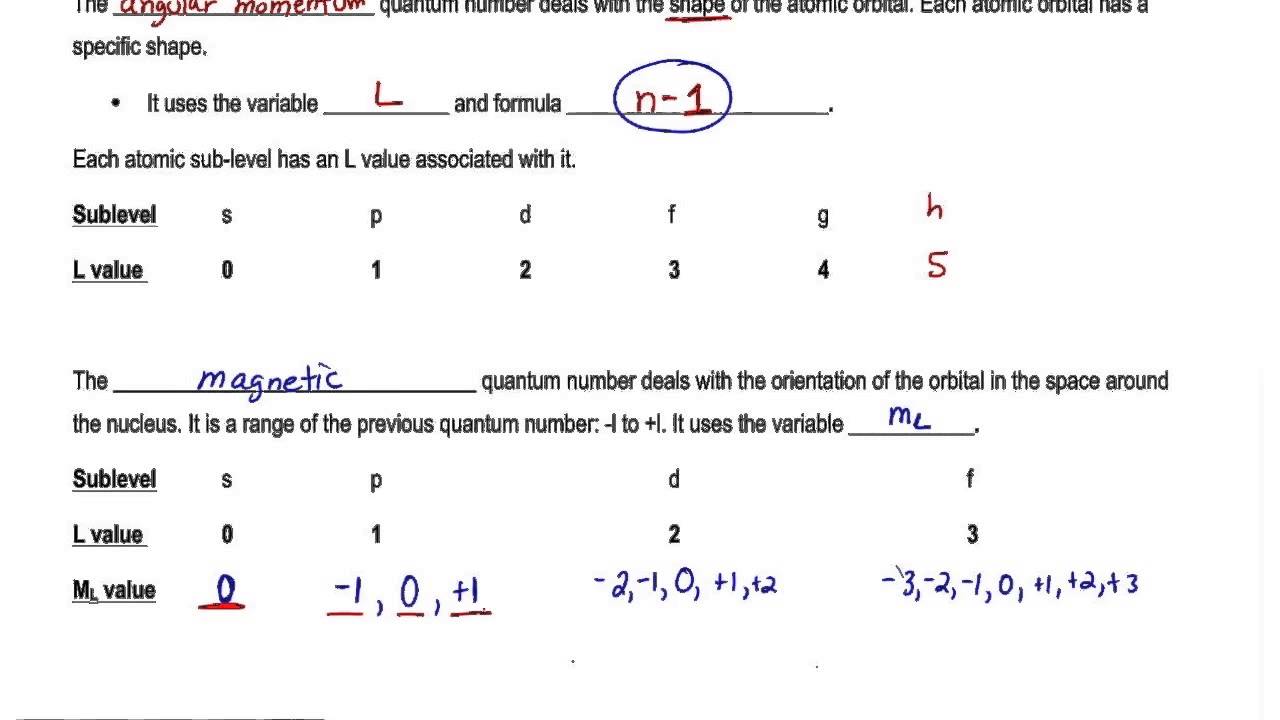
Azimuthal or Angular Momentum l. 1 2 1 2 2 4 3 2 9. The magnetic quantum number is denoted by the letter m or ml and for a given value of l it can have all the values ranging from -l to l including zero. Principal Quantum Number n.
Principal Quantum Number n Principal quantum number determines the size of the orbital. Since the spin can be 12 or 12 there are two combinations. Quantum Mechanics and Quantum Numbers post provides complete information about quantum numbers with examples and wave function. Spin can either be 12 or -12.
Principal quantum number n 3. It is denoted by the letter textn and can only have an integral value except zero ie textn 1234. This is denoted by m l. It describes orientation of orbital in space under magnetic field which obtained due to angular momentum of electron and thus it relates to the value of l.
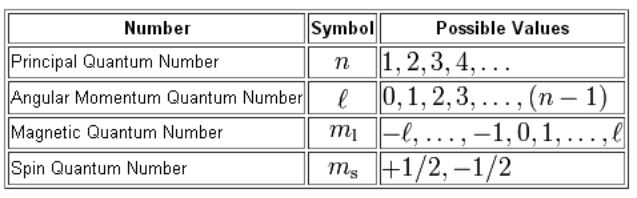
M 2 l 1 values of l 1 to -1 including zero. In that theory n automatically determined the orbits energy content but this rule is no longer valid for a multielectronic atom. The Spin Quantum Number m_s describes the angular momentum of an electron. An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the Schrödinger wave equation for the hydrogen atom.
Principle quantum number is one of the most critical quantum numbers that tells about an electrons principal energy level orbit or shell. N 2 l 1 m l 0 m s. Magnetic quantum number formula. There is one orbital in an s subshell l 0 three orbitals in a p subshell l 1 and five orbitals in a d subshell l 2.
What does the Magnetic Quantum Number Determine. However some quantities still take on continuous values as well see. The descriptor quantum arises because in contrast with classical mechanics certain quantities take on only discrete values. N 1237 it corresponds to the quantum number of the Bohr model.
In later sections we will explore the significance of each of the quantum numbers. The first three n l m l specify the particular orbital of interest and the fourth m s specifies how many electrons can occupy that orbital. Azimuthal quantum number l 2. There are four quantum numbers.
For a given value of the azimuthal quantum number the magnetic quantum numbers can have any integral value between 1 to -1. Magnetic quantum number ml 2 1 0 1 2. Consequently it is dependent on the orbital angular momentum. 0123 n-1 Magnetic Quantum Number.
An electron spins around an axis and has both angular momentum and orbital angular momentum. The larger n is the greater the distance from the nucleus. The magnetic quantum number identifies the orientation of shapes of electron orbitals with respect to a given direction usually that of a strong magnetic field. A quantum number is a value that is used when describing the energy levels available to atoms and molecules.
Quantum numbers are constants which identify each solution to Schrödingers. We also tabulate some of the simpler solutions. Magnetic quantum number m l l1 l2. In quantum mechanics particles have wavelike properties and a particular wave equa-.
N 1 2 3 8. What is the formula of principal quantum number. The magnetic quantum number determines the number of preferred orientation of the electron present in a sub shell. Calculate the minimum and maximum number of electrons which have magnetic quantum number m 1 and spin quantum number s 12 in chromium.
An equation given by Schrodinger which has a better physical interpretation in terms. Quantum Mechanics and Quantum Numbers. For every value of l m has 2l 1 values. Magnetic quantum number m.
Each electron in an atom is described by four different quantum numbers. The magnetic quantum number primarily determines the number of orbitals and the orientation of orbitals in a given sub-shell. Each l value represents a specific orbit named after the description of the hydrogen spectrum such s for sharp l 0 p for principal l 1 d for diffuse l 2 f for fundamental l 3 etc. The number of orbitals in a shell is the square of the principal quantum number.
Source Image @ physics.stackexchange.com
Source Image @ study.com
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ www.youtube.com
Source Image @ study.com
Source Image @ byjus.com
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ www.priyamstudycentre.com
Source Image @ www.sciencedirect.com
If you re looking for Quantum Number Formulas you've arrived at the ideal place. We have 10 images about quantum number formulas including images, photos, pictures, wallpapers, and much more. In these page, we also have variety of images available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.
If the posting of this site is beneficial to your suport by sharing article posts of the site to social media marketing accounts you have such as for example Facebook, Instagram among others or can also bookmark this website page with all the title Principal Quantum Number An Overview Sciencedirect Topics Use Ctrl + D for laptop devices with Glass windows operating system or Control + D for personal computer devices with operating-system from Apple. If you are using a smartphone, you can also utilize the drawer menu on the browser you utilize. Be it a Windows, Mac, iOs or Android os operating system, you'll be in a position to download images using the download button.





0 comments:
Post a Comment