Quantum number formula ~ Magnetic quantum numbers can have a total of 2 ℓ 1 values. Principal quantum number n 3. Indeed recently has been hunted by users around us, perhaps one of you. People are now accustomed to using the internet in gadgets to see image and video information for inspiration, and according to the title of this post I will discuss about Quantum Number Formula Calculate the minimum and maximum number of electrons which have magnetic quantum number m 1 and spin quantum number s 12 in chromium.
Source Image @ hyperphysics.phy-astr.gsu.edu
Quantum Numbers And Atomic Energy Levels

The most important thing to. The Schrodinger equation is difierent in a few ways from the other wave equations weve seen in this book. Your Quantum number formula image are available. Quantum number formula are a topic that has been searched for and liked by netizens now. You can Find and Download or bookmark the Quantum number formula files here
Quantum number formula - The magnetic quantum number can be derived from solving the azimuthal equation of the hydrogen Schrodinger equation. The number of orbitals in a subshell is therefore 2l 1. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. Section 103 we discuss a number of examples.
Spin can either be 12 or -12. Magnetic quantum number ml 2 1 0 1 2. There are 2l1 orbitals in each subshell. For s-orbital ℓ 0.
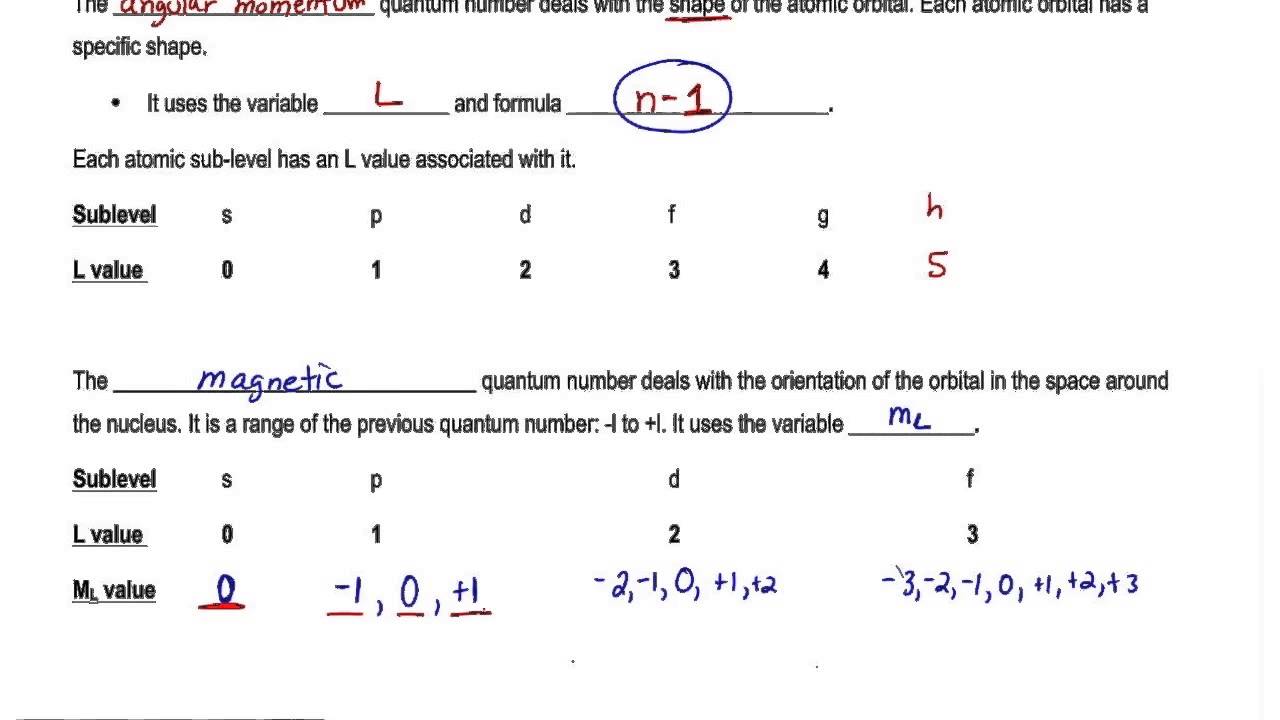
For a solution of either the nonrelativistic Pauli equation or the relativistic Dirac equation the quantized angular momentum see angular momentum quantum number can be written as. Quantum Mechanics and Quantum Numbers. There is one orbital in an ssubshell l 0 three orbitals in a psubshell l 1 and five orbitals in a dsubshell l 2. Values of are from zero to n-1.
For f-orbital ℓ 3. In quantum mechanics particles have wavelike properties and a particular wave equa-tion the Schrodinger equation governs how these waves behave. The quantum number refers to the projection of the angular momentum in this arbitrarily-chosen direction conventionally called the -direction or quantization axis. Magnetic quantum number formula.
For p-orbita ℓ 1. The magnetic quantum number determines the number of preferred orientation of the electron present in a sub shell. Magnetic quantum number m. Quantum Mechanics and Quantum Numbers post provides complete information about quantum numbers with examples and wave function.
Consequently it is dependent on the orbital angular momentum. What does the Magnetic Quantum Number Determine. N 2 l 1 m l 0 m s. Magnetic Quantum Number m l.
Since the spin can be 12 or 12 there are two combinations. L z displaystyle L_z the magnitude of the angular momentum in the z displaystyle z. Each l value represents a specific orbit named after the description of the hydrogen spectrum such s for sharp l 0 p for principal l 1 d for diffuse l 2 f for fundamental l 3 etc. 12 1 22 4 32 9.
For d-orbital ℓ 2. Azimuthal quantum number l 2. M l -l 0 l. This is denoted by m l.
This number divides the subshell into individual orbitals which hold the electrons. Angular momentum quantum number l n 1. The magnetic quantum number is denoted by the letter m or ml and for a given value of l it can have all the values ranging from -l to l including zero. What is the formula of principal quantum number.
The Magnetic quantum number given orbital angular momentum formula is defined as the number which divides the subshell into individual orbitals which hold the electrons and is represented as m cosϑsqrtl l1 or magnetic_quantum_number cosThetasqrtAzimuthal Quantum Number Azimuthal Quantum Number1. For a given value of the azimuthal quantum number the magnetic quantum numbers can have any integral value between 1 to -1. For a given value of ℓ magnetic quantum number can have values ranging from l to l. S s s 1 ℏ displaystyle Vert mathbf s Vert sqrt ss1hbar.
Azimuthal quantum number describes the shape of orbital. For every value of l m has 2l 1 values. The fourth quantum number is independent of the first three allowing the first three quantum numbers of two electrons to be the same. Quantum mechanics takes into account the dual behavior of matter.
So it can be a positive integer zero and a negative integer. The number of orbitals in a shell is the square of the principal quantum number. Hence the number of values of m is 2l 1. Magnetic quantum number m l l1 l2.
The magnetic quantum number is denoted by the letter m or m l and the value for a given value of l is in the range of -l to l including zero. With the help of the value of azimuthal quantum number we can determine the total number of energy sub-levels in a given energy level. M 2 l 1 values of l 1 to -1 including zero. An equation given by Schrodinger which has a better physical interpretation in terms.
Thus the s subshell has only one orbital the p subshell has three orbitals and so on. An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the Schrödinger wave equation for the hydrogen atom. The magnetic quantum number determines the number of preferred orientations of the electrons present in a subshell. It is denoted by.
The magnetic quantum number primarily determines the number of orbitals and the orientation of orbitals in a given sub-shell. It describes orientation of orbital in space under magnetic field which obtained due to angular momentum of electron and thus it relates to the value of l. There are four quantum numbers. The magnetic quantum number identifies the orientation of shapes of electron orbitals with respect to a given direction usually that of a strong magnetic field.
Specifies the orientation in space of an orbital of a given energy n and shape l.
Source Image @ study.com
Source Image @ www.youtube.com
Source Image @ www.pinterest.com
Source Image @ study.com
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ www.azwc1.org
Source Image @ www.priyamstudycentre.com
Source Image @ www.youtube.com
Source Image @ www.sciencedirect.com
If you are searching for Quantum Number Formula you've arrived at the ideal place. We have 10 images about quantum number formula including pictures, photos, photographs, wallpapers, and much more. In such page, we also have number of images available. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.
If the posting of this web site is beneficial to your suport by sharing article posts of this site to social media accounts you have such as Facebook, Instagram and others or may also bookmark this blog page together with the title Principal Quantum Number An Overview Sciencedirect Topics Make use of Ctrl + D for laptop or computer devices with House windows operating-system or Control + D for laptop devices with operating system from Apple. If you are using a smartphone, you can even use the drawer menu with the browser you utilize. Be it a Windows, Apple pc, iOs or Android operating system, you'll still be in a position to download images utilizing the download button.






0 comments:
Post a Comment