Quantum no. formula ~ Magnetic quantum number formula. For a given value of the azimuthal quantum number the magnetic quantum numbers can have any integral value between 1 to -1. Indeed lately is being searched by consumers around us, perhaps one of you personally. Individuals are now accustomed to using the internet in gadgets to see image and video data for inspiration, and according to the title of this post I will talk about about Quantum No. Formula Magnetic quantum number m.
Source Image @ study.com
Magnetic Quantum Number Definition Example Video Lesson Transcript Study Com

This is denoted by m l. The four quantum numbers used to describe the electrons are n2 ℓ1 m1 0 or -1 and s12 the electrons have parallel spins. Your Quantum no. formula photographs are ready. Quantum no. formula are a topic that has been hunted for and liked by netizens today. You can Get or bookmark the Quantum no. formula files here
Quantum no. formula - For the outer valence electrons of a carbon atom the electrons are found in the 2p orbital. A quantum number beginning in n 3ℓ 0 describes an electron in the s orbital of the third electron shell of an atom. 12 1 22 4 32 9. A maximum number of two electrons.
The fact that the quantum potential is a necessary part of this new mathematical derivation of the origin of the universe is fascinating. The spin quantum number tells us the orientation of an electron within an orbital and has two possible values. The total angular momentum s is given by. It describes orientation of orbital in space under magnetic field which obtained due to angular momentum of electron and thus it relates to the value of l.
Cutting Edge Quantum Formulas Nikola Tesla 1866--1943 The day science begins to study non-physical phenomena it will make more progress in one decade than in. The magnetic quantum number can be derived from solving the azimuthal equation of the. To its wave nature. However boundaries or constraints need to be put in place to match the equations with the physical world.
It explains the presence of holes and the transport of holes and electrons in electronic devices. This is yet one more introductory course on quantum computing. Thanks to this the phenomenon of quantum entanglement is described which in practice states that. The spin quantum number is S n 2 n is said to be a non-negative integer.
Quantum yield is calculated using the equation 4 Φ A Φ S F A F S O D S O D A where F stands for the integrated fluorescence intensity OD is the optical density or absorbance at 360 nm and the subscripts A and S stand for arrestin and standard respectively. After this the wave equation gets solved to find these modes then the electron gets the energy by the frequency of the model and by Einsteins Quantum Equation that is E hv. If the proximate electrons have an opposite spin direction and the magnetic field created by them cancels each other and no magnetic field is existent. Sn n1 h.
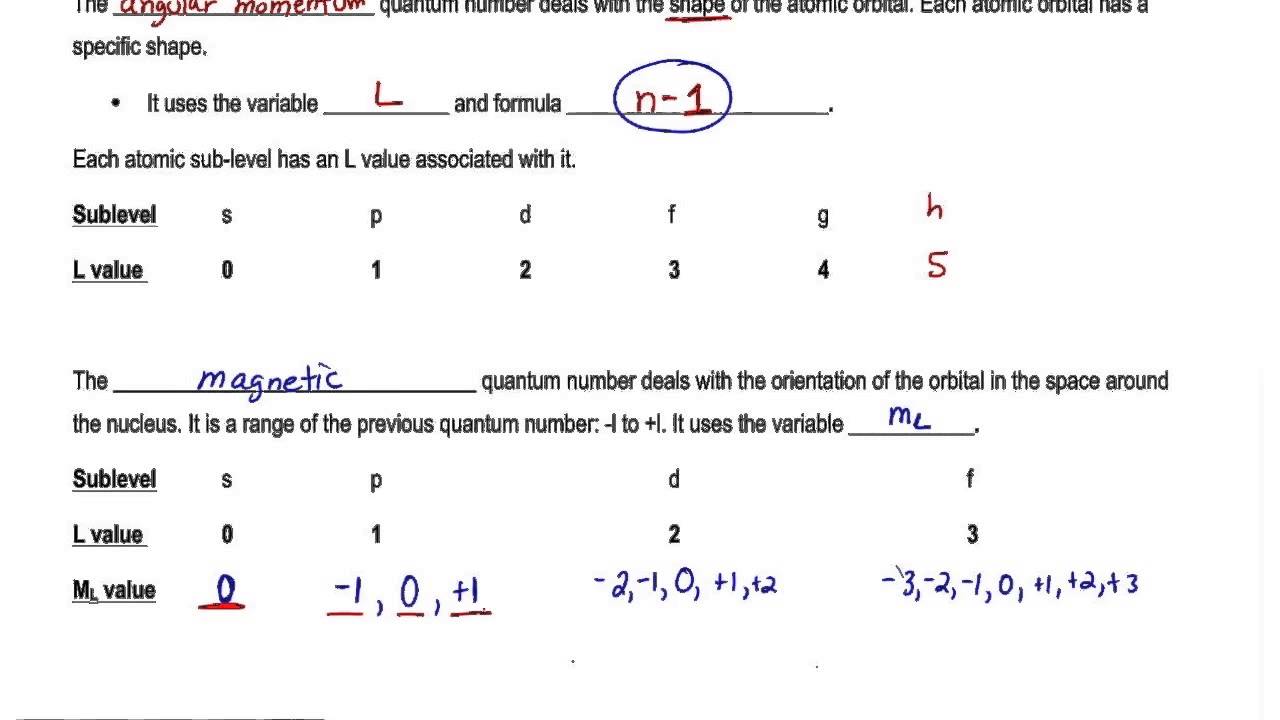
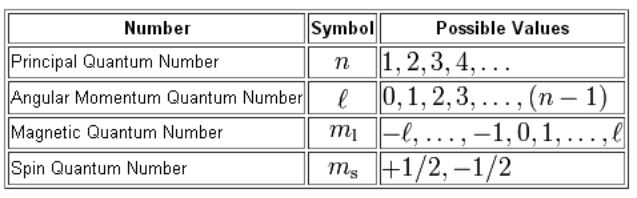
Ms 12 for spin up and ms -12 for spin down. Principal Quantum Number n. F ma which is actually a second-order differential equation m d2x dt2 dV dx 11 It is useful to reexpress this second-order equation as a pair of first-order equations dx dt p m dp dt dV dx 12 where m is the mass and p is the momentum of the baseball. There is one orbital in an ssubshell l 0 three orbitals in a psubshell l 1 and five orbitals in a dsubshell l 2.
Quantum mechanics can also explain the radiation of hot body and its change of color with respect to temperature. Principle quantum number is one of the most critical quantum numbers that tells about an electrons principal energy level orbit or shell. The number of orbitals in a shell is the square of the principal quantum number. The different energy levels of atoms were identified with the simple vibrational modes of the wave equation.
On the first week I try to explain in simple language I hope where the computational power of. Quantum Number Example. The above equation is that of Dirac and is the most beautiful equation known in physics. He wrote down a wave equation the so-called Schrodinger equation that governs how the waves evolve in space and time.
The magnetic quantum number identifies the orientation of shapes of electron orbitals with respect to a given direction usually that of a strong magnetic field. Specifies the energy of an electron and the size of the orbital the distance from the nucleus of the peak in a radial probability distribution plot. The number of orbitals in a subshell is therefore 2l 1. Even though the equation is correct the correct interpretation of what the wave.
M 2 l 1 values of l 1 to -1 including zero. It is denoted by the letter textn and can only have an integral value except zero ie textn 1234. N 1 2 3 8. Here I concentrate more on how the mathematical model of quantum computing grows out from physics and experiment while omitting most of the formulas when possible and rigorous proofs.
5 The LSZ Reduction Formula 3 49 6 Path Integrals in Quantum Mechanics 57 7 The Path Integral for the Harmonic Oscillator 6 63 8 The Path Integral for Free Field Theory 3 7 67 9 The Path Integral for Interacting Field Theory 8 71 10 Scattering Amplitudes and. The azimuthal quantum number can also denote the number of angular nodes present in an orbital. How to find Magnetic Quantum Number. The values of the spins are 0 12 1 32 2 etc.
Well deal with this equation in depth below. Quantum mechanics has played an important role in photonics quantum electronics and micro-electronics. Quantum numbers in general and magnetic quantum numbers in particular can be derived while solving the Schrodinger equati on. In chemistry this quantum number is very important since it specifies the shape of an atomic orbital and strongly influences chemical bonds and bond angles.
We want to find the solution of these equations such that xt 0 X in and xt 0 t X. Erwin Schrodinger formulated a version of quantum mechanics that was based on waves.
Source Image @ www.youtube.com
Source Image @ www.priyamstudycentre.com
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ www.reddit.com
Source Image @ byjus.com
Source Image @ study.com
Source Image @ www.sciencedirect.com
Source Image @ chemistrybytes.com
If you are looking for Quantum No. Formula you've arrived at the ideal place. We ve got 10 images about quantum no. formula adding images, photos, pictures, wallpapers, and more. In these page, we additionally provide number of images available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.
If the publishing of this webpage is beneficial to our suport by sharing article posts of the site to social media marketing accounts that you have such as Facebook, Instagram among others or can also bookmark this blog page with all the title Quantum Numbers And Schrodinger S Wave Equation Chemistrybytes Com Make use of Ctrl + D for pc devices with Glass windows operating-system or Command + D for laptop devices with operating system from Apple. If you are using a smartphone, you can also use the drawer menu in the browser you use. Be it a Windows, Mac, iOs or Android operating-system, you'll be able to download images utilizing the download button.





0 comments:
Post a Comment