Gcse science empirical formula questions ~ The empirical formula is Fe 2 O 3. A periodic table will be required to complete this practice test. Indeed lately is being hunted by consumers around us, maybe one of you personally. Individuals now are accustomed to using the net in gadgets to see image and video information for inspiration, and according to the title of the article I will discuss about Gcse Science Empirical Formula Questions Work out the formula of aluminium sulphide.
Source Image @ www.tes.com
Rfm By Mass And Empirical Formula Calculations Teaching Resources

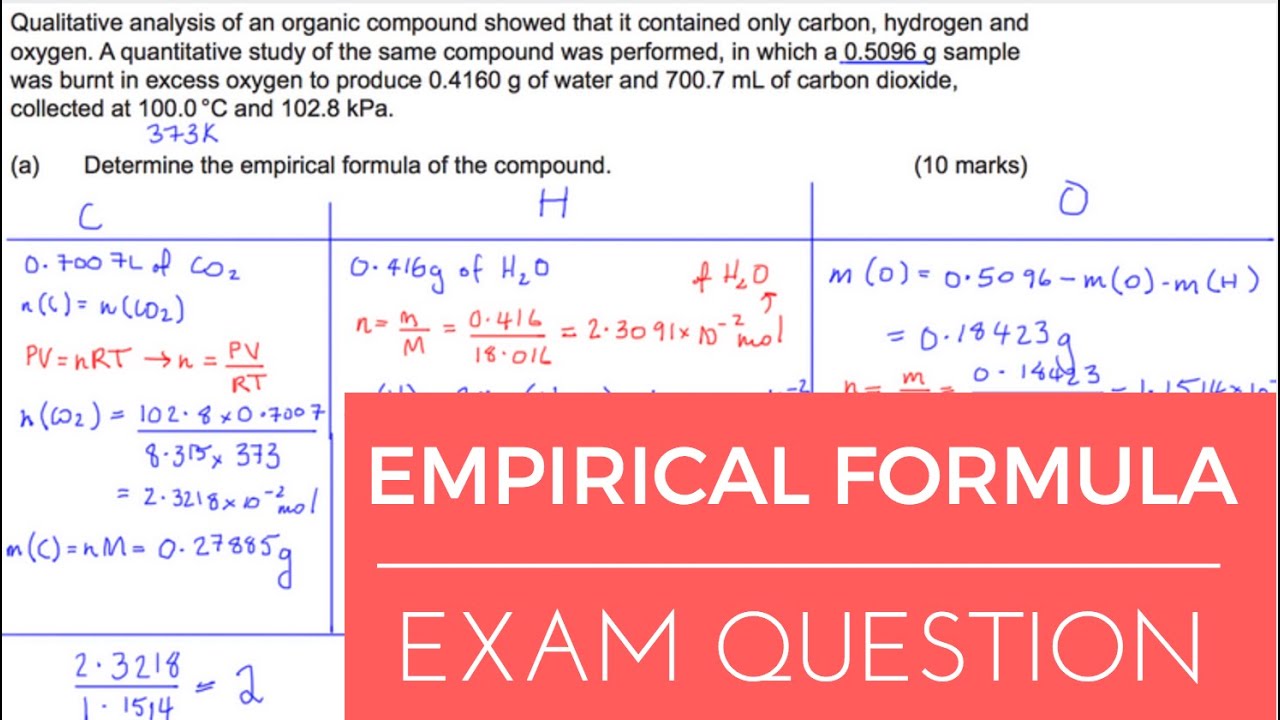
Empirical Formula Practice Test Questions The empirical formula of a compound represents the simplest whole-number ratio between the elements that make up the compound. Empirical Formula GCSE Science AQA C2 Edexcel OCR link to exam questions - YouTube. Your Gcse science empirical formula questions photos are ready in this website. Gcse science empirical formula questions are a topic that has been searched for and liked by netizens now. You can Find and Download or bookmark the Gcse science empirical formula questions files here
Gcse science empirical formula questions - X y. What is the molecular formula of X. IGCSE Edexcel Chemistry Paper 2 predictions. Step 2 Divide relative formula mass of X by the relative empirical mass.
Organic molecules often have different empirical and molecular formulae. The empirical formula of ethanoic acid is CH 2 O. Amount of sulphur combined with the aluminium 150 - 54 96g. Relative atomic masses A r.
O 16 Na 23 P 31. Which Compound has 3g of Carbon and 05g of Hydrogen if the RMM is 42. The topics and specification points covered within these lessons include. RMM of the compound 42.
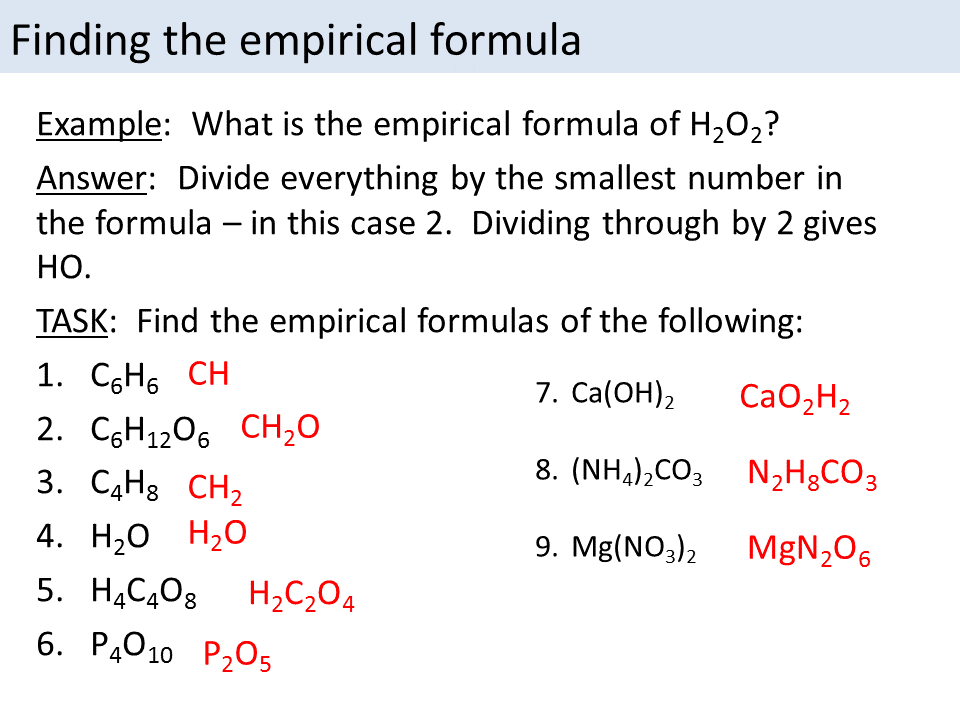
This is because we can divide each number in C 6. Empirical Formula from Reacting Masses. The empirical formula is the simplest whole number ratio of the atoms of each element present in one molecule or formula unit of the compound. State the empirical formula for each of the following compounds.
Step 3 Represent the ratio into the form M x O y Eg MgO. 336g of Iron reacts with 144g of Oxygen. 42 14 3 3 CH 2. What is the formula of Iron Oxide.
20g of Ca reacts with 355g of Cl. Empirical formula worksheet gcse. Moles a A r b A r. The empirical formula for a compound is C 2 H 5.
The molecular formula of ethanoic acid is C 2 H 4 O 2. What does Empirical mean. Empirical formula is MgO. The PowerPoint and accompanying resources have been designed to cover points 144 145 of the Edexcel GCSE Chemistry specification and also covers those points in the Chemistry section of the Combined Science course.
Calculate the empirical formula of sodium phosphate. Finally students apply this to 2 exam style empirical formula questions. Calculate the empirical formula of the compound. The empirical formula of X is C 4 H 10 S 1 and the relative formula mass of X is 180.
3 Divide the relative molecular mass of the compound by the relative molecular mass of the empirical formula. It is calculated from knowledge of the ratio of masses of each element in the compound. The percent composition of a compound was found to be 635 silver 82 nitrogen and 283 oxygen. An empirical formula gives the simplest whole number ratio of atoms of each element in the compound.
For example the molecular formula of glucose is C 6 H 12 O 6 but the empirical formula is CH 2 O. To gain full marks you must show your working. 180 90 2. This 10-question practice test deals with finding empirical formulas of chemical compounds.
5 Something went wrong please try again later. Multiply the numbers in the empirical formula by the factor 3. Providing quality GCSE revision resources to help you get the grade you need. A sample of sodium phosphate contains 345 g of sodium 155 g of phosphorus and 320 g of oxygen.
Worksheet 8 empirical formulas 1. Creative Commons Sharealike Review. This is because we can divide each number in C 6. Calculate the empirical formula of copper iodide.
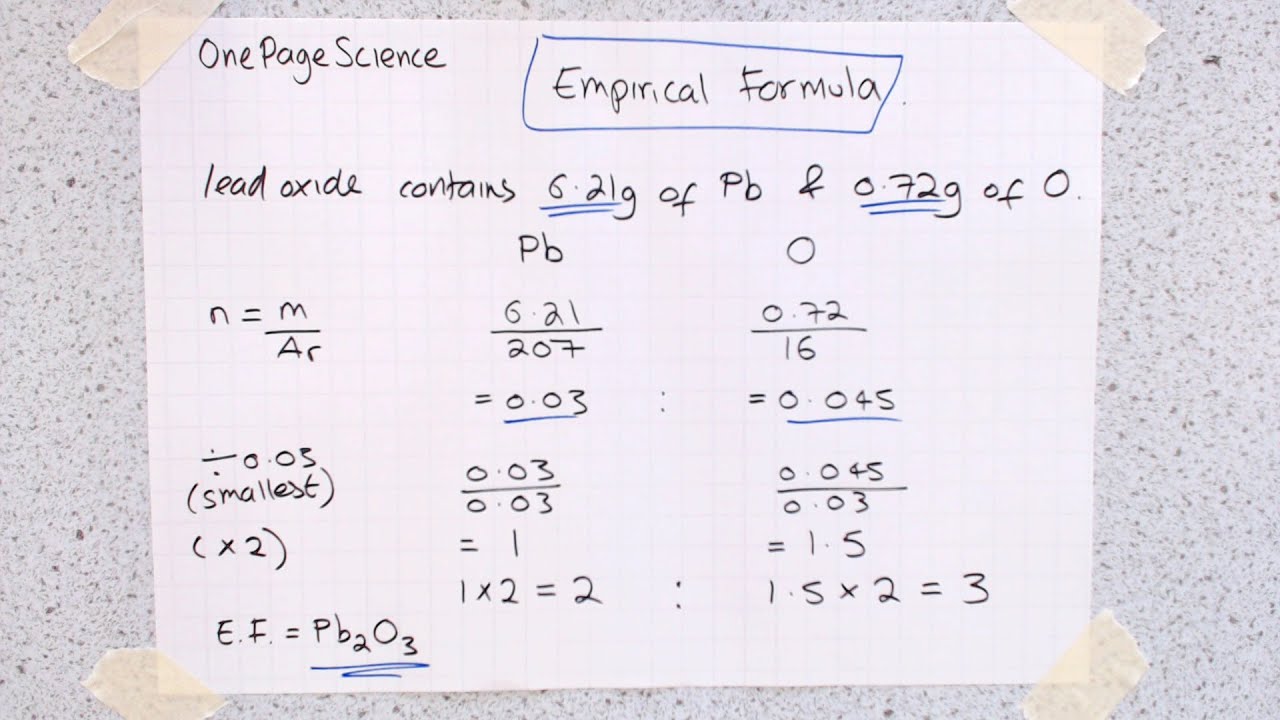
GCSE SCIENCE CHEMISTRY HIGH SCHOOL The empirical formula is CH 2. It is found that 54g of aluminium forms 150g of aluminium sulphide. Since iron oxide is an ionic compound the actual formula is the same as the empirical formula. Empirical formula calculation Example 53 The empirical formula of aluminium sulfide.
Step 1 Divide each of the two masses by the relative atomic masses of the elements. For example the molecular formula of glucose is C 6 H 12 O 6 but the empirical formula is CH 2 O. In an experiment 127 g of hot copper reacts with iodine vapour to form 381 g of copper iodide. This lesson describes how the empirical formula of a compound can be deduced from the masses of the different parts.
Empirical formula. The Empirical Formula of a Compound. Writing word equations Writing balanced symbol equations with state symbols Calculate relative formula masses Know that the mole is the unit for the amount of a substance Understand how to carry out calculations involving amount relative atomic and formula mass Calculate reacting. Calculating the empirical formula from a reaction.
4 Total 5 marks Page 3 of 9. What is the formula of Calcium Chloride. Al 27 and S 32. RMM of the empirical formula RMM of CH 2 12 2 x 1 14.
Step 1 Calculate the relative empirical formula mass C x 4 H x 10 S x 1 12 x 4 1 x 10 32 x 1 90. Molecular formula C 3 H 6. Empirical formula for GCSE Chemistry. Find the empirical formula and name for each of the following.
C2 AQA GCSE Empirical Formula and Reacting Masses Questions. Step 2 Simplify the ratio.
Source Image @ www.youtube.com
Source Image @ www.youtube.com
Source Image @ www.tes.com
Source Image @ www.savemyexams.co.uk
Source Image @ www.tes.com
Source Image @ www.youtube.com
Source Image @ www.tes.com
Source Image @ www.savemyexams.co.uk
Source Image @ www.tes.com
If you re looking for Gcse Science Empirical Formula Questions you've arrived at the right place. We have 10 graphics about gcse science empirical formula questions including images, photos, pictures, backgrounds, and more. In such webpage, we additionally provide number of images available. Such as png, jpg, animated gifs, pic art, logo, black and white, transparent, etc.
If the posting of this website is beneficial to your suport by discussing article posts of the site to social media accounts which you have such as for example Facebook, Instagram among others or can also bookmark this blog page with all the title Empirical Formula Exam Questions With Mark Scheme Teaching Resources Work with Ctrl + D for personal computer devices with Glass windows operating-system or Demand + D for pc devices with operating-system from Apple. If you use a smartphone, you can even use the drawer menu in the browser you utilize. Whether its a Windows, Macintosh, iOs or Android operating-system, you'll be able to download images using the download button.






0 comments:
Post a Comment