Formula of quantum number ~ Magnetic_quantum_number 2Azimuthal Quantum Number1 Go. According to the questions the principal quantum number n 4. Indeed recently has been searched by consumers around us, maybe one of you. People are now accustomed to using the net in gadgets to see video and image information for inspiration, and according to the title of this article I will discuss about Formula Of Quantum Number N 2 l 1 m l 0 m s 1 2.
Source Image @ www.youtube.com
Differential Equations 39 Quantum Numbers Youtube

12 1 22 4 32 9. It is denoted by the letter textn and can only have an integral value except zero ie textn 1234. Your Formula of quantum number pictures are ready. Formula of quantum number are a topic that has been hunted for and liked by netizens now. You can Get or bookmark the Formula of quantum number files here
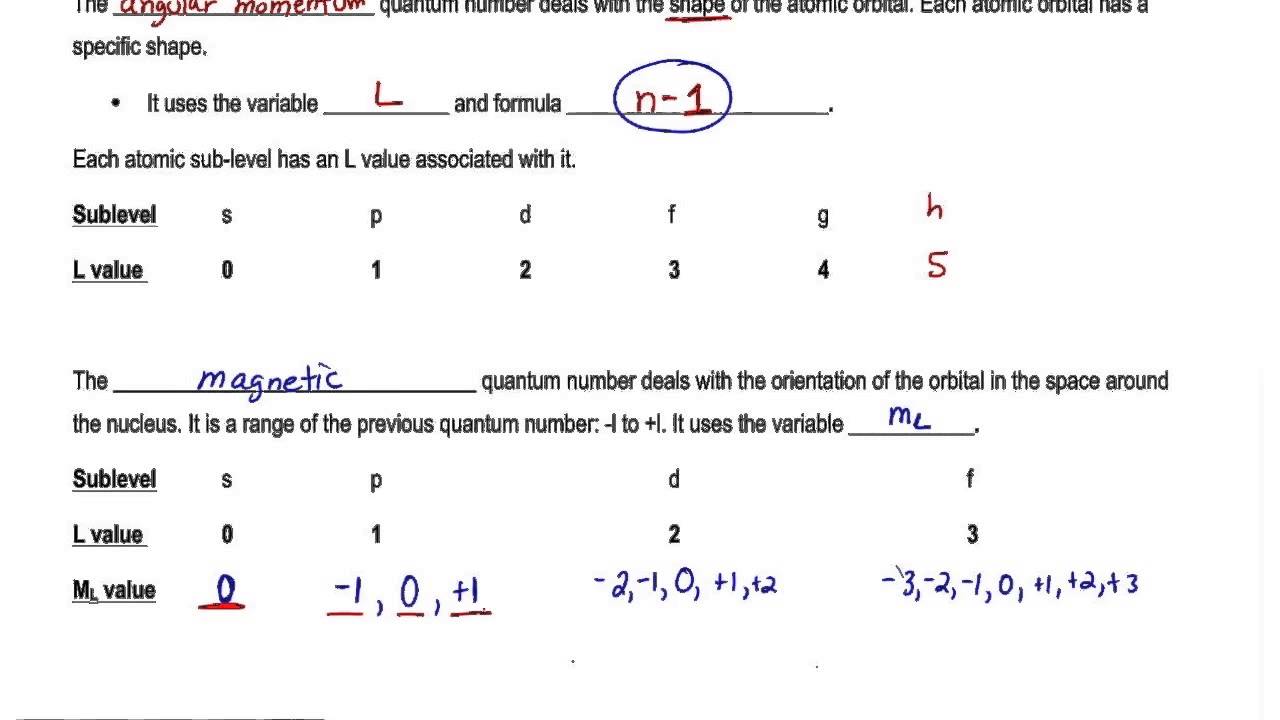
Formula of quantum number - Maximum number of electrons in sub shell of magnetic quantum number. Magnetic quantum number m. The number of orbitals in a shell is the square of the principal quantum number. The Magnetic quantum number given orbital angular momentum formula is defined as the number which divides the subshell into individual orbitals which hold the electrons is calculated using magnetic_quantum_number cos Theta sqrt Azimuthal Quantum.
The particles having integral value 012 of spin are called bosons. The magnetic quantum number can be derived from solving the azimuthal equation of the hydrogen Schrodinger equation. Consequently it is dependent on the orbital angular momentum quantum number also known as the. L 2 Ψ ℏ 2 ℓ ℓ 1 Ψ displaystyle mathbf L 2Psi hbar 2ell ell 1Psi.
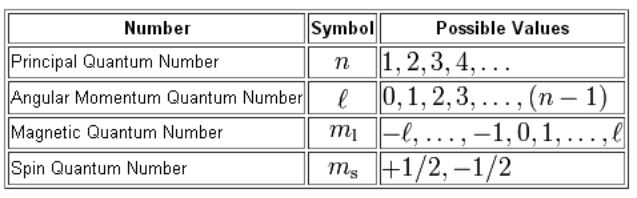
M l - ℓ to ℓ -101 Spin Quantum Number. Specifies the energy of an electron and the size of the orbital the distance from the nucleus of the peak in a radial probability distribution plot. There is one orbital in an ssubshell l 0 three orbitals in a psubshell l 1 and five orbitals in a dsubshell l 2. 0123 n-1 Magnetic Quantum Number.
Where n 1 2 3. The principal quantum number n represents the. An electron spins around an axis and has both angular momentum and orbital angular momentum. The fourth quantum number is independent of the first three allowing the first three quantum numbers of two electrons to be the same.
N l and m. The number of orbitals in a subshell is therefore 2l 1. The magnetic quantum number is denoted by the letter m or ml and for a given value of l it can have all the values ranging from -l to l. Each type of subatomic particle has fixed spin quantum numbers like 012 1 32.
Therefore l which is the angular quantum number can have the values 0123 and not beyond 3. Since the spin can be 12 or 12 there are two combinations. Principal quantum number n 3. The spin value of an electron proton neutron is 12.
Because angular momentum is a vector the Spin Quantum Number s has both a magnitude 12 and direction or -. M s 12 -12. The particles having half integral value 12 32 of spin are called fermions. What does the Magnetic Quantum Number Determine.
Number of orbitals of magnetic quantum number in main energy level. Magnetic quantum number ml 2 1 0 1 2. The spin may lie in 2s12 orientation. Azimuthal quantum number l 2.
Number_of_electron 2 2Azimuthal Quantum Number1 Go. Principal Quantum Number n. The magnetic quantum number primarily determines the number of orbitals and the orientation of orbitals in a given sub-shell. An atomic electrons angular momentum L is related to its quantum number ℓ by the following equation.
Calculate the minimum and maximum number of electrons which have magnetic quantum number m 1 and spin quantum number s 12 in. And is called the principal quantum number and h is Plancks constantThis formula is not correct in quantum mechanics as the angular momentum magnitude is described by the azimuthal quantum number but the energy levels are accurate and classically they correspond to the sum of potential and kinetic energy of the electron. N 2 l 1 m l 0 m s 1 2. M 2 l 1 values of l 1 to -1 including zero.
Principle quantum number is one of the most critical quantum numbers that tells about an electrons principal energy level orbit or shell. The Spin Quantum Number m_s describes the angular momentum of an electron. The Total magnetic quantum number value formula is defined as the number which divides the subshell into individual orbitals which hold the electrons and is represented as m 2l1 or magnetic_quantum_number 2Azimuthal Quantum Number1. Total_number_of_orbitals Number of orbit2 Go.
The magnetic quantum number determines the number of preferred orientation of the electron present in a sub shell. The principal quantum number is n. N 1 2 3 8. There are three quantum numbers.
It describes orientation of orbital in space under magnetic field which obtained due to angular momentum of electron and thus it relates to the value of l.
Source Image @ byjus.com
Source Image @ www.priyamstudycentre.com
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ www.youtube.com
Source Image @ chemistrybytes.com
Source Image @ study.com
Source Image @ study.com
Source Image @ www.youtube.com
If you are looking for Formula Of Quantum Number you've come to the right location. We have 10 graphics about formula of quantum number adding pictures, photos, photographs, backgrounds, and much more. In such webpage, we also have number of images out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.
If the publishing of this internet site is beneficial to our suport by discussing article posts of the site to social media accounts as such as Facebook, Instagram and others or may also bookmark this blog page along with the title The Magnetic Quantum Number Ml Youtube Work with Ctrl + D for pc devices with Home windows operating-system or Control + D for computer devices with operating system from Apple. If you use a smartphone, you can even use the drawer menu from the browser you use. Be it a Windows, Macintosh, iOs or Android operating-system, you'll still be in a position to download images utilizing the download button.





0 comments:
Post a Comment