Magnetic spin quantum number formula ~ The spin quantum number tells us the orientation of an electron within an orbital and has two possible values. M 2 l 1 values of l 1 to -1 including zero. Indeed recently is being searched by consumers around us, maybe one of you personally. People now are accustomed to using the internet in gadgets to view image and video information for inspiration, and according to the name of the article I will discuss about Magnetic Spin Quantum Number Formula The z-component is quantized in units of taking values 12.
Source Image @ www.priyamstudycentre.com
Quantum Number Orbital Definition Formula Diagram Shape

In general the spin quantum number is denoted with an s. The probability of rotation in one direction is 12. Your Magnetic spin quantum number formula photos are ready. Magnetic spin quantum number formula are a topic that has been searched for and liked by netizens now. You can Get or bookmark the Magnetic spin quantum number formula files here
Magnetic spin quantum number formula - The spin quantum number is 12. Nonetheless the magnetic moment associated with electron spin is also 1µ B. Values of are from zero to n-1. This is a component of the atomic electrons total orbital angular momentum whose magnitude is related to the azimuthal quantum number of its subshell by the equation.
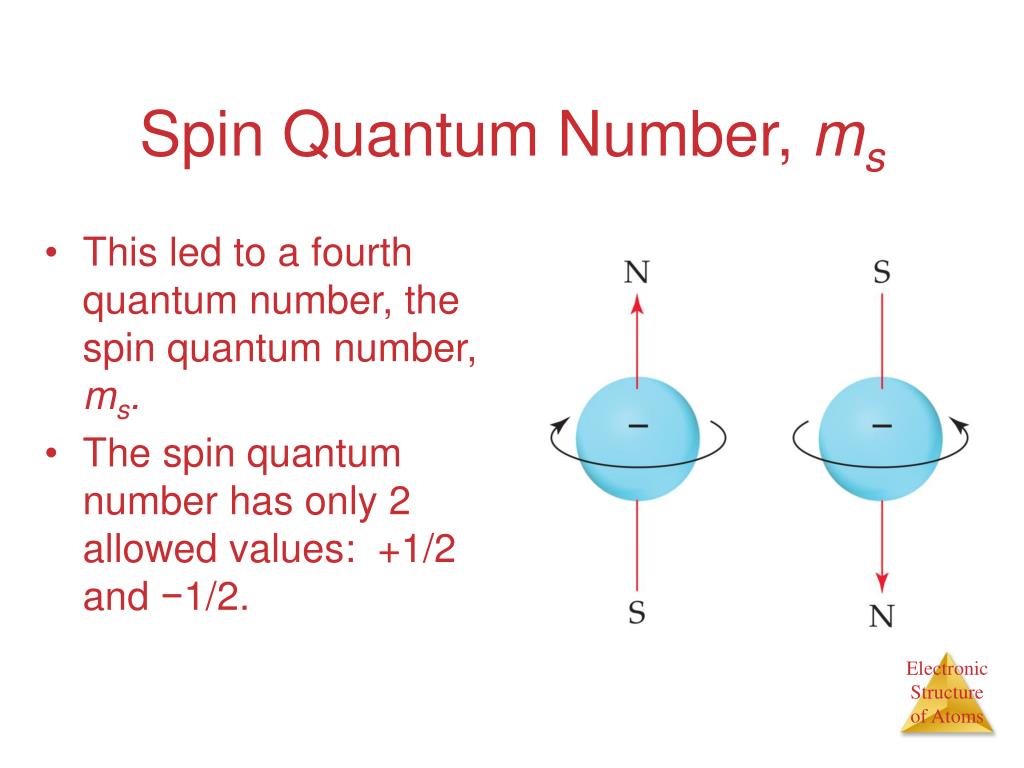
The value of s for an elementary particle depends only on the type of particle and cannot be altered in any known way in contrast to the spin direction described below. The magnetic quantum number identifies the orientation of shapes of electron orbitals with respect to a given direction usually that of a strong magnetic field. Spin is a consequence of relativistic quantum mechanics. A maximum number of two electrons.
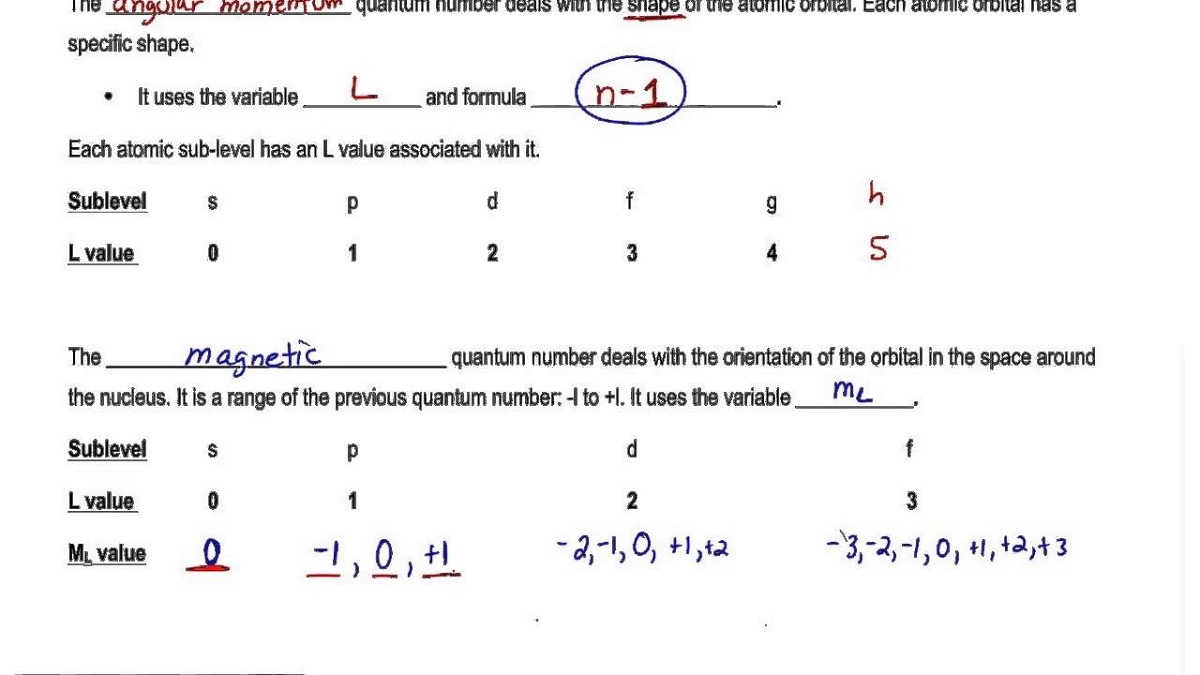
For d-orbital ℓ 2. Magnetic quantum number formula. Therefore the spin quantum number can only have two values. What does the Magnetic Quantum Number Determine.
Orbitals that have same value of principal quantum number form a Shell n. This formula is related to the number of unpaired electrons n by the equation μs n n2 BM. It is denoted by. However generally the first unpaired electron in each orbital is usually assigned the 12 magnetic spin value.
The electron has a mysterious built-in spin angular momentum. For p-orbita ℓ 1. The spin of an electron can be represented by either -12 or 12. S total spin quantum number.
Of unpaired electrons magnetic moment μs total spin quantum number S. Quantum spin precession in a magnetic field Last lecture we saw that the electron had a magnetic moment µ orbit e 2me Lˆ due to orbital degrees of freedom. It describes orientation of orbital in space under magnetic field which obtained due to angular momentum of electron and thus it relates to the value of l. The magnetic quantum number is calculated by rmm -l to rml The spin quantum number has two values only 12 and 12 Learn About Principal Quantum.
The conventional definition of the spin quantum number is s n 2 where n can be any non-negative integer. The spin angular momentum is s. Hence the allowed values of s are 0 1 2 1 3 2 2 etc. The Spin Angular Momentum of an object is defined as the angular momentum about its centre of mass coordinate and is represented as L sqrt s s 1 h 2 pi or angular_momentum sqrt Spin Quantum Number Spin Quantum Number 1 Plancks Constant 2 pi.
L 1 d. The magnetic quantum number can be derived from solving the azimuthal equation of the hydrogen Schrodinger equation. For a given value of the azimuthal quantum number the magnetic quantum numbers can have any integral value between 1 to -1. For nuclei of spin quantum number I 1 2 placed in an external magnetic field B 0 there are two available energy levels two quantum statesthey are associated with the magnetic spin quantum numbers m I 1 2.
If you have any queries drop a comment below and we will get back to you. For s-orbital ℓ 0. For an anticlockwise spin the value -12 is assigned for spin quantum number. For the magnetic spin quantum number Im pretty sure that it doesnt matter which electron you make 12 or -12.
The intrinsic electron spin imparts an additional contribution µ spin γSˆ where the gyromagnetic ratio γ g e 2m e and g known as the Lande g-factor is very. As with all charged particles if the nucleus is moved in a loop it will generate a magnetic field. Magnetic quantum number m. We hope this article on Quantum Number has helped you.
The magnetic quantum number primarily determines the number of orbitals and the orientation of orbitals in a given sub-shell. For f-orbital ℓ 3. L 2 f. With the help of the value of azimuthal quantum number we can determine the total number of energy sub-levels in a given energy level.
L 0 p. If l 2 m can be -2 -1 0 1 or 2. When the electron spins in a clockwise direction the value 12 is assigned for the spin quantum number. For all electrons however s ½.
Spin quantum number s indicates the direction of spin of electrons. An associated number ms gives the possible orientations of s in the same way ml gave the possible orientations of l. L ℏ ℓ ℓ 1 displaystyle Lhbar sqrt ell ell 1. The magnetic quantum number m can be any integer between - l and l.
Magnetic quantum number left m_l right determines the orientation and number of orbitals. Ms 12 for spin up and ms -12 for spin down. The possible values of ms are integer increments between. Orbitals within the shells are divided into subshell l s.
Spin Quantum Number describes the angular momentum of an electron Plancks Constant is the. M s -em es. Azimuthal quantum number describes the shape of orbital. Spin magnetic moment-If magnetic moment arise due to the spin of unpaired electrons then μs 4s s1 BM.
We have now established that the nucleus has spin which can be denoted using specific quantum numbers. The magnetic moment mu is related to the angular momentum of the nucleus by mugamma I. This is denoted by m l. The angular quantum number l determines the shape of an orbital in which electrons are present.
Consequently it is dependent on the orbital angular momentum.
Source Image @ study.com
Source Image @ unacademy.com
Source Image @ www.slideserve.com
Source Image @ www.pinterest.com
Source Image @ study.com
Source Image @ slideplayer.com
Source Image @ www.youtube.com
Source Image @ www.world-today-news.com
Source Image @ defitioni.blogspot.com
If you re looking for Magnetic Spin Quantum Number Formula you've arrived at the right place. We have 10 graphics about magnetic spin quantum number formula adding images, photos, photographs, wallpapers, and more. In such web page, we also provide number of images available. Such as png, jpg, animated gifs, pic art, symbol, black and white, transparent, etc.
If the posting of this website is beneficial to our suport by spreading article posts of the site to social media marketing accounts which you have such as Facebook, Instagram among others or may also bookmark this website page while using title Definition Of Magnetic Quantum Number In Chemistry Defitioni Make use of Ctrl + D for laptop devices with Glass windows operating system or Command line + D for computer devices with operating-system from Apple. If you are using a smartphone, you can also use the drawer menu of the browser you utilize. Be it a Windows, Mac, iOs or Android os operating-system, you'll still be able to download images utilizing the download button.





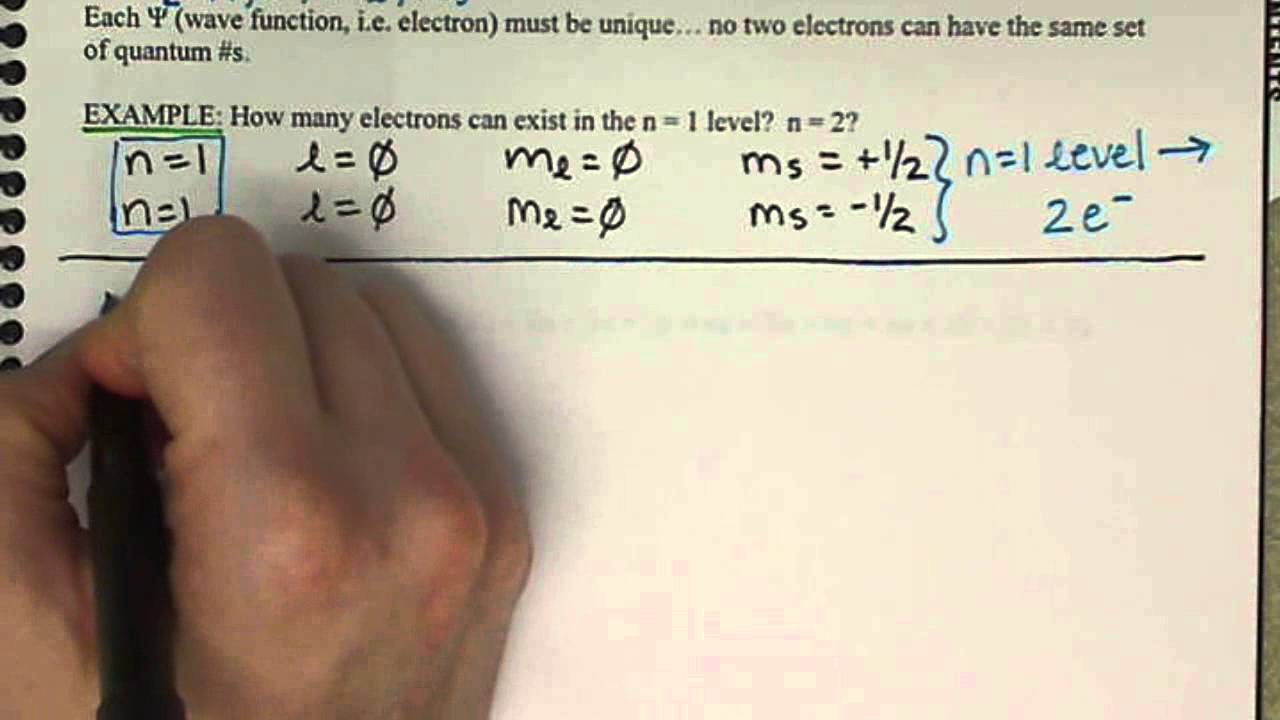

0 comments:
Post a Comment