Fluorescence quantum yield formula ~ The fluorescence quantum yield is defined as the ratio of the number of photons emitted to the number of photons absorbed. Quantum yields when measured using direct and indirect excitation. Indeed lately has been hunted by consumers around us, perhaps one of you. Individuals now are accustomed to using the net in gadgets to see image and video information for inspiration, and according to the name of the post I will talk about about Fluorescence Quantum Yield Formula It can be shown that this definition leads to where k f is the fluorescence rate constant and k i denotes the rate constants of all the decay processes from the first excited state of the fluorophore.
Source Image @ www.aatbio.com
Fluoroquest Fluorescence Quantum Yield Determination Kit Optimized For Bioconjugates Aat Bioquest
Fro calculating quantum yield here i have given some link from that you will get clear information about analysis of fluorescence spectrum analysis. Chi-ninbisulfate in 005 M Sulfuric acid will be provided as reference. Your Fluorescence quantum yield formula photographs are ready in this website. Fluorescence quantum yield formula are a topic that is being searched for and liked by netizens now. You can Get or bookmark the Fluorescence quantum yield formula files here
Fluorescence quantum yield formula - The IS detector is equipped with an internal cuvette holder so that absorbance measure-ments can be performed with the cuvette inside the IS. There are two methods for calculating the fluorescence quantum yield - single. Experimentally relative fluorescence quantum yields can be determined by measuring fluorescence of a fluorophore of known quantum yield. Explain your choice of the solution concentration.
Anthracene quantum yield is 027 in ethanol2 and tris22-bipyridylrutheniumII. Fluorescence quantum yield is defined as the ratio of the number of molecules that fluoresce to the total number of excited molecules or the ratio of photons emitted to photons absorbed see Eq. C -1 - A E. The gradients of the graphs obtained in 4.
In addition the spectropho-tometer has a cuvette holder outside the IS. Therefore the fluorescence quantum yield of the unknown is obtained from the product of the quantum yield of the reference and the quotient of the two gradients91112 Method Qs Qr 3 ms ns 2 mr nr Qs r 2 Ar Es ns 2 As Er nr. Phi dfrack_fsum_i k_i labelEq1 where k_f is the rate of spontaneous emission of radiation and the denominator is the sum of all rates of excited state decay for each deactivation process ie phosphorescence intersystem crossing internal conversion. 2 with k R 0158 10 9 s 1 mean of all seven k R values Table 1 and k nR described by Eqs 3336.
This is achieved by calculating the quantum. First the two standard compounds are crosscalibrated using this equation. Above are proportional to the quantum yield of the different samples. Calculation of Fluorescence Quantum Yields from Acquired Data.
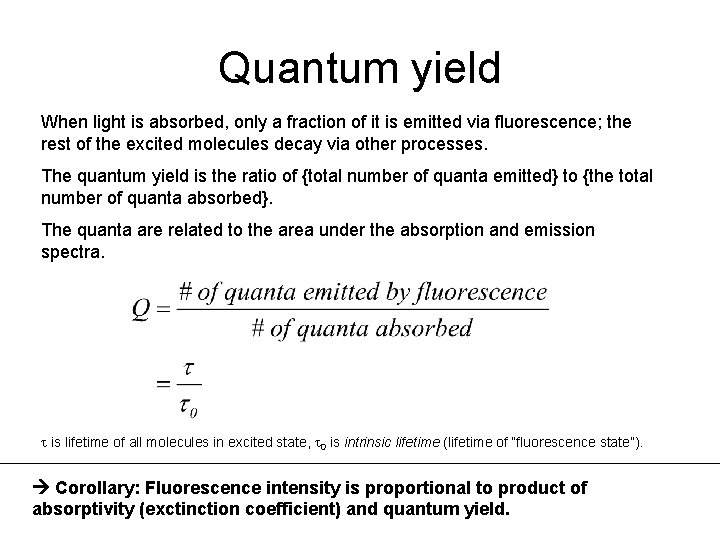
4Determine the emission quantum yield of a 9-anthracenecarbonitrile solution. Quantum Yield 3 E. There is a method that uses fluorescence lifetimes and different concentrations of a quencher to calculate the quantum yield of a molecule. Conversion into an absolute quantum yield is achieved through the equation given in the text.
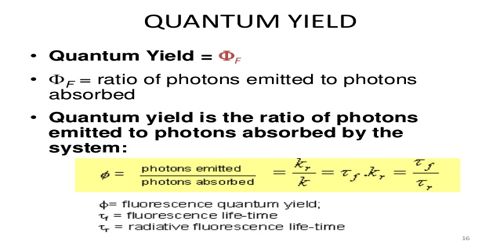
Another way to define the fluorescence quantum yield is by the excited state decay rates. Fluorophores with the largest quantum yields approaching unity exhibit the brightest emissions. Φ N p h o t o n s e m i t t e d N p h o t o n s a b s o r b e d displaystyle Phi frac rm N_ photons emitted rm N_ photons absorbed. Fluorescence quantum yield QY using a commercial spectrophotometer with a 150 mm integrating sphere IS detector.
Where Q is the quantum yield I is the integrated intensity n is the refractive index and OD is the optical density. The fluorescence quantum yield QY of a dye is the fraction of photons absorbed resulting in emission of fluorescence. A A 1 - L. The Quantum Yield for the de Mello method is calculated using Equation 3 and uses Emptysolvent blank Indirect and Direct spectra much like the spectra shown in Figure 3 for fluorescein.
The quantum yield is calculated using Eq. Knowledge of it is important for the successful development of fluorometric indication and visualization methods and for the understanding of light-driven processes in the natural. The fluorescence quantum yield is defined as the ratio of the number of photons emitted to the number of photons absorbed1. They are related by the quantum yield equation given below.
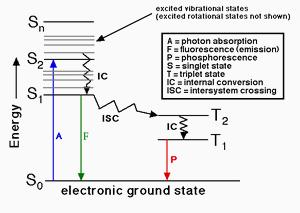
Fluorescence quantum yields Ф were estimated by integrating the area under the fluorescence curves using the equation1 Where A was the area under the fluorescence spectral curve OD was optical density of the compound at the excitation wavelength and η was the refractive indices of the solvent. φNO Quantum yield of non-regulated non-photochemical energy loss in PS II equivalent to YNO φNPQ Quantum yield of regulated non-photochemical energy loss in PS II equivalent to YNPQ F Fluorescence yield measured briefly before application of a Saturation Pulse Fm Maximal fluorescence yield of dark-adapted sample with all PS II centers. The fluorescence quantum yield Φ f is a key property that characterizes the ability of a fluorophore to convert absorbed photons into emitted photons under various environmental conditions. However a more accurate method to determine quantum yields is to prepare solutions with optical densities in the range of 01001 and to.
The equation on the right is used where t f is the quantum yield and k f k nr and k t are the rate constants of fluorescence non-radiative dissipation and energy transfer respectively τ f is the fluorescence lifetime of the sample. The sub script R refers to the reference fluorophore of known quantum yield. 5aCalculate the mass m of naphthaline which you have to weight in to get a stock solution of 1 M in 10 ml acetonitrile. As one can see for each fixed E y value the quantum yield first decreases as E x increases then reaches a minimum and then starts to increase.
Fluorescence quantum yield PLQY no. Fluorescence quantum yield is defined as the ratio of the number of molecules that fluoresce to the total number of excited molecules or the ratio of photons emitted to photons absorbed see Eq. Absolute values are calculated using the standard samples which have a fixed and known fluorescence quantum yield value according to the following equation. Dfrack_fk_fk_ik_eck_ick_pdk_d Using this equation as an example to explain fluorescence a high fluorescence rate k f value and low values of the all the other relative rate constant terms k f k i k ec k ic k pd k d will give a large phi which suggest that.
Source Image @ www.horiba.com
Source Image @ www.researchgate.net
Source Image @ www.azom.com
Source Image @
Source Image @ slidetodoc.com
Source Image @ qsstudy.com
Source Image @ www.youtube.com
Source Image @ www.researchgate.net
Source Image @ www.horiba.com
If you re looking for Fluorescence Quantum Yield Formula you've come to the perfect place. We ve got 10 images about fluorescence quantum yield formula including images, photos, photographs, wallpapers, and much more. In these webpage, we also have variety of images available. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.
If the posting of this webpage is beneficial to our suport by posting article posts of the site to social media accounts which you have such as for example Facebook, Instagram among others or may also bookmark this website page along with the title What Are Luminescence Quantum Yields Horiba Make use of Ctrl + D for pc devices with House windows operating-system or Control + D for pc devices with operating system from Apple. If you use a smartphone, you can even use the drawer menu on the browser you use. Be it a Windows, Macintosh personal computer, iOs or Android os operating system, you'll be able to download images utilizing the download button.


.jpg)



0 comments:
Post a Comment