Empirical formula ks3 science ~ It should be used following my lesson on Formula Mass and Empirical Formulae. Molecular Formula textn times Empirical Formula is the general relationship between the empirical and molecular formulas. Indeed recently is being hunted by consumers around us, maybe one of you personally. Individuals now are accustomed to using the net in gadgets to view video and image information for inspiration, and according to the title of this article I will discuss about Empirical Formula Ks3 Science The simplest formula or the empirical formula provides the lowest whole number ratio of atoms existent in a compound.
Source Image @ www.tes.com
Empirical Formula Gcse And A Level Teaching Resources
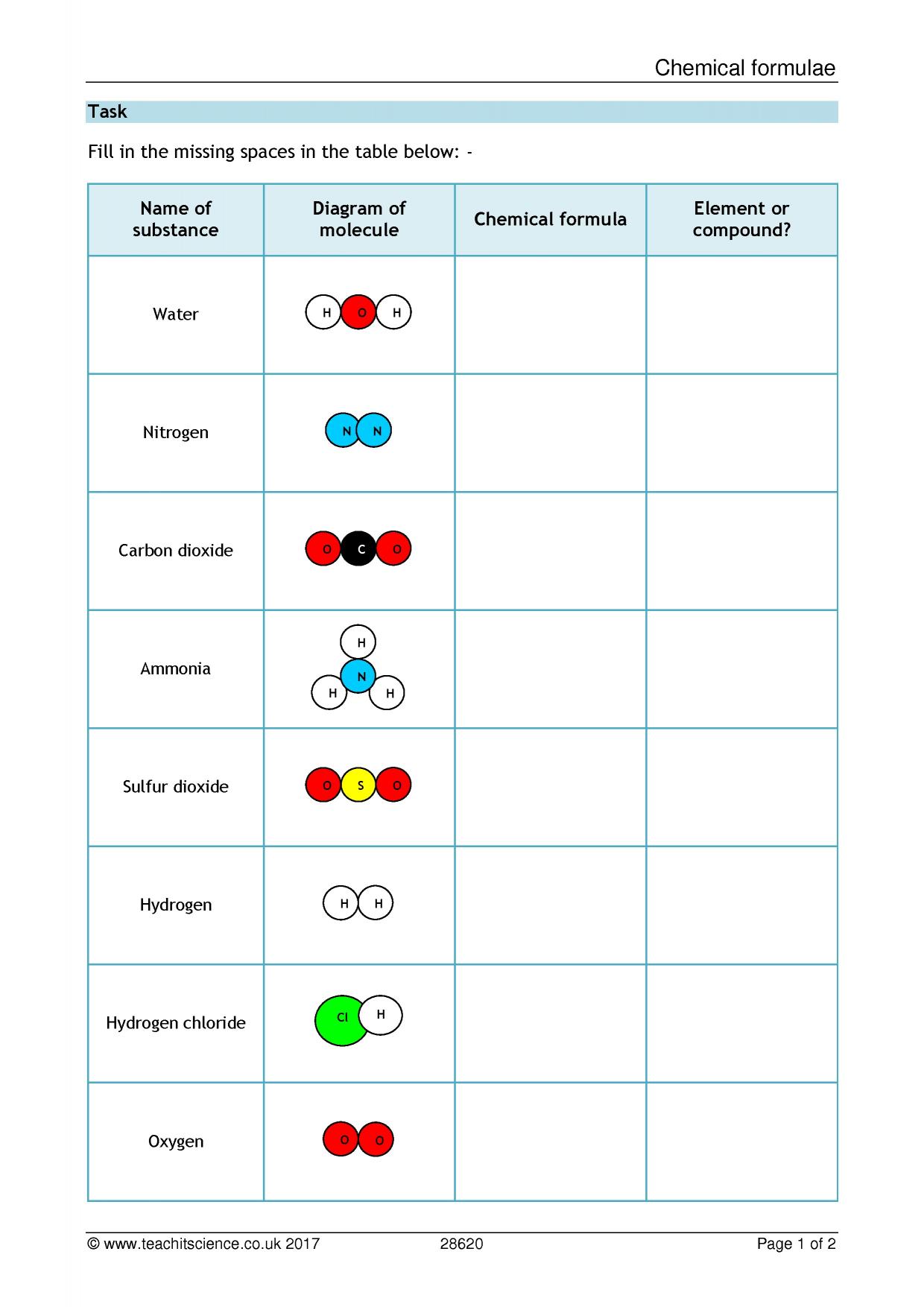
Of a compound is the simplest whole number ratio of atoms of each element in a compound. The empirical formula of benzene is CH hydrogen peroxide is HO Glucose is CH 2 O. Your Empirical formula ks3 science photographs are available in this site. Empirical formula ks3 science are a topic that is being hunted for and liked by netizens now. You can Find and Download or bookmark the Empirical formula ks3 science files here
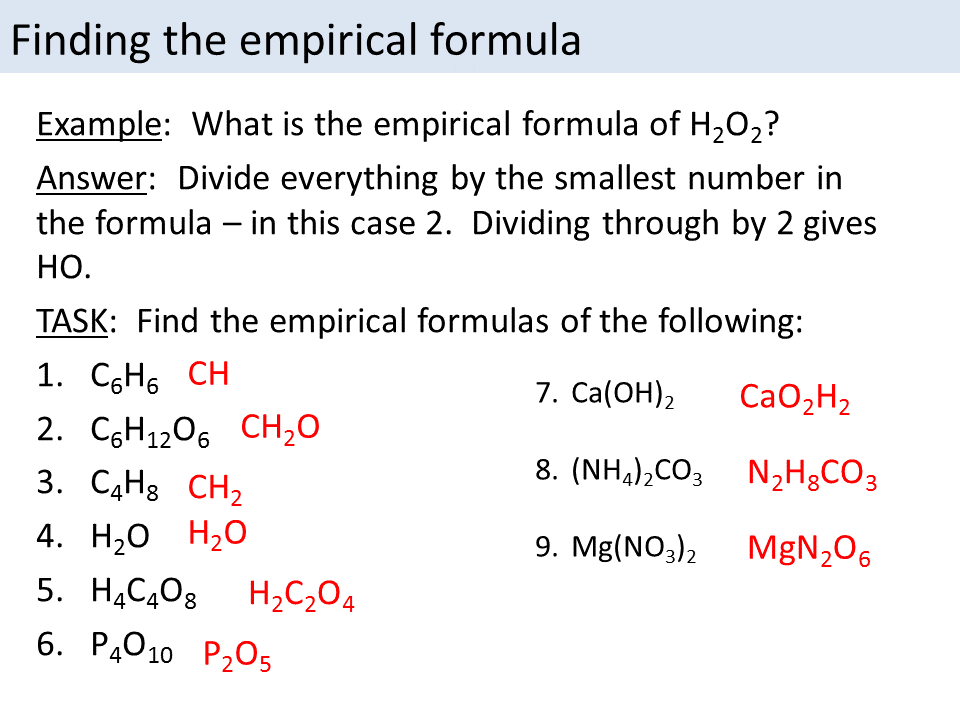
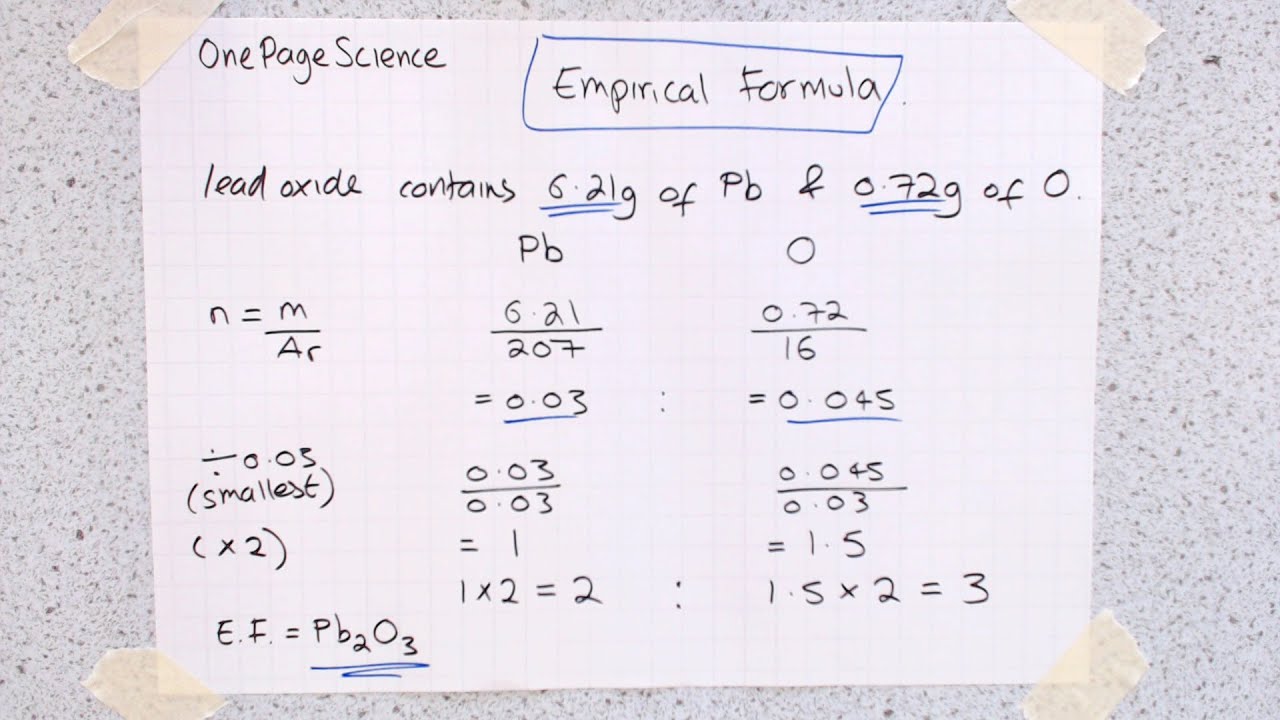
Empirical formula ks3 science - The simplest ratio of atoms of each element in a compound is called the empirical formula. Steps for Determining an Empirical Formula. As with most stoichiometry problems it is necessary to work in moles. Because of the law of conservation of mass the empirical formula is often found using elemental composition or mass percentage.
Learn the basics about Empirical formulae from percentage composition. Let Mr Thornton simplify how to calculate the Empirical Formula of a compound - it. Carbon dioxide units contain one carbon atom and two oxygen atoms. The molecular formula contains information on the actual number of atoms.
Mass is conserved in chemical reactions. It shows the relative number of atoms of each element. Determine the empirical formula of a compound containing. Reaction information is shown using word and symbol equations.
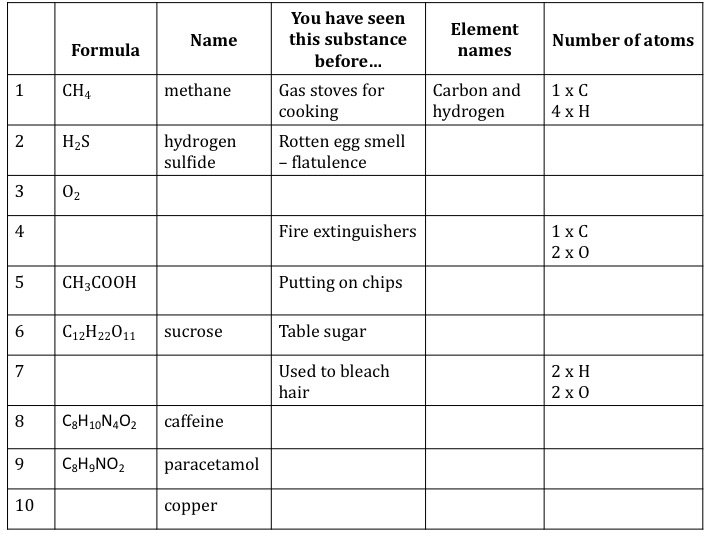
In literature Koutsoyiannis et al 1998 it is stated that it can formulate a generalized IDF relationship between rainfall intensity I T rainfall duration t d and return period T r in the form of power-law relation as. A secondary education revision video to help you pass your Science GCSE. Find the empirical formula of a compound that is 4838 carbon 812 hydrogen and 535 oxygen by mass. What is an empirical formulae.
The empirical formula of a substance represents the simplest relative whole number ratio of the atoms of each element contained in the molecule of the substance. Empirical and Molecular Formula The empirical formula of a compound is the chemical formula which expresses the simplest whole number ratio of the atoms of the various elements present in one molecule of the compound. For example CH2O is the empirical formula of Glucose C6H12O6. Mass is conserved in chemical reactions.
The ratios are denoted by subscripts next to the element symbols. 5 I T fn T r fn t d which has the advantage of a separable functional dependence of I T on t d and T r. What they wouldve observed is the ratio of the atoms to one another in a molecule without knowing in the exact molecule how many of that atom there are. It is determined using data from experiments and therefore empirical.
Empirical and Molecular Formulae. Is the simplest whole number ratio of atoms. Molecular formulas show all atoms of each element in a molecule. The empirical formula is a chemical formula that represents the simplest ratio of atoms in the chemical formula of the compound.
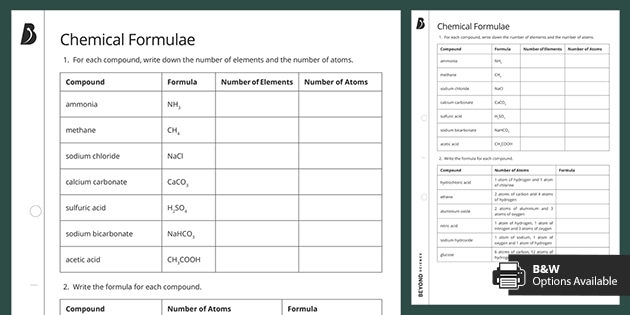
The resource meets all required specification points for the Edexcel 9-1 GCSE Chemistry course. The empirical formula of a compound is defined as the formula that shows the ratio of elements present in the compound but not the actual numbers of atoms found in the molecule. Empirical Formula Molecular Formula - There are two broad classes of the formula called Empirical formula Molecular formula. Its chemical formula is written as CO 2.
Visit BYJUS to learn more about it. So the empirical formula tells you what people have observed maybe before they even knew that there was such a thing as atoms. The empirical formula of a compound. Empirical formulas worksheet answer key.
Empirical formula The empirical formula is the simplest formula for a compound in which atoms of different elements are present in simple ratio. Empirical IDF formula. Chemistry Single Science Quantitative chemistry. However we need to put that in terms of our 4 molar solution so we divide by 4 to get 220 which is 010 L or 100 mL of the NaOH solution.
The empirical formula for glucose is CH 2 O. Over 200 resources available for KS3-KS4 Science KS5 Chemistry and Whole School. The empirical formula is the simplest version of a chemical formula for example C3H8. The reaction stoichiometry indicates that for every 3 moles of hydrogen gas we need 6 moles of sodium hydroxide so we need ⅖ mol NaOH.
The molecular formula shows the actual number of atoms of each element present in a. The empirical formula is a simple type of chemical formula which provides the smallest whole-number ratio among elements within a molecular compound. Thus the molecule of glucose contains atoms of carbon hydrogen and oxygen in the ratio 121. How do you calculate a percentage composition.
It is accessible for students easy to follow for teachers and is appropriate for Combined and Triple Science classes Foundation and Higher tiers. Some of the worksheets for this concept are empirical and molecular formula work empirical and molecular formulas work empirical and molecular formula work percent composition and molecular formula work work 8 empirical formulas h o n o 4i empirical and molecular formula work. Lesson resources are suitable for live lessons in school remote teaching at home or independent student study. The simplest ratio of atoms of each element in a compound is called the empirical formula.
The simplest ratio of the atoms present in a molecule. The empirical formula shows the simplest ratio of elements in a compound also called simple formulas. Sometimes you see more complex formulae such as Na 2 SO 4 and FeOH 3. The relative number of atoms of every element in the compound is provided by this formula.
Source Image @ thescienceteacher.co.uk
Source Image @ www.twinkl.de
Source Image @ www.teachit.co.uk
Source Image @ www.tes.com
Source Image @ www.tes.com
Source Image @ www.tes.com
Source Image @ www.tes.com
Source Image @ slideplayer.com
Source Image @ www.youtube.com
If you are searching for Empirical Formula Ks3 Science you've reached the perfect location. We ve got 10 images about empirical formula ks3 science including pictures, pictures, photos, wallpapers, and much more. In such webpage, we additionally provide number of images out there. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.
If the posting of this web site is beneficial to your suport by posting article posts of the site to social media marketing accounts which you have such as Facebook, Instagram among others or may also bookmark this blog page while using title Empirical Formula Calculations Gcse Aqa C2a Youtube Use Ctrl + D for computer devices with Home windows operating system or Command word + D for computer devices with operating system from Apple. If you are using a smartphone, you can also use the drawer menu on the browser you utilize. Whether its a Windows, Mac pc, iOs or Google android operating system, you'll still be in a position to download images utilizing the download button.




0 comments:
Post a Comment