Uncertainty science formula ~ It applies to predictions of future events to physical measurements that are already made or to the unknown. Calculate the square of each sample minus the mean. Indeed recently is being searched by users around us, maybe one of you. People now are accustomed to using the internet in gadgets to see video and image information for inspiration, and according to the name of the post I will discuss about Uncertainty Science Formula Uncertainty of 1 mm.
Source Image @ www.wikihow.com
3 Ways To Calculate Uncertainty Wikihow
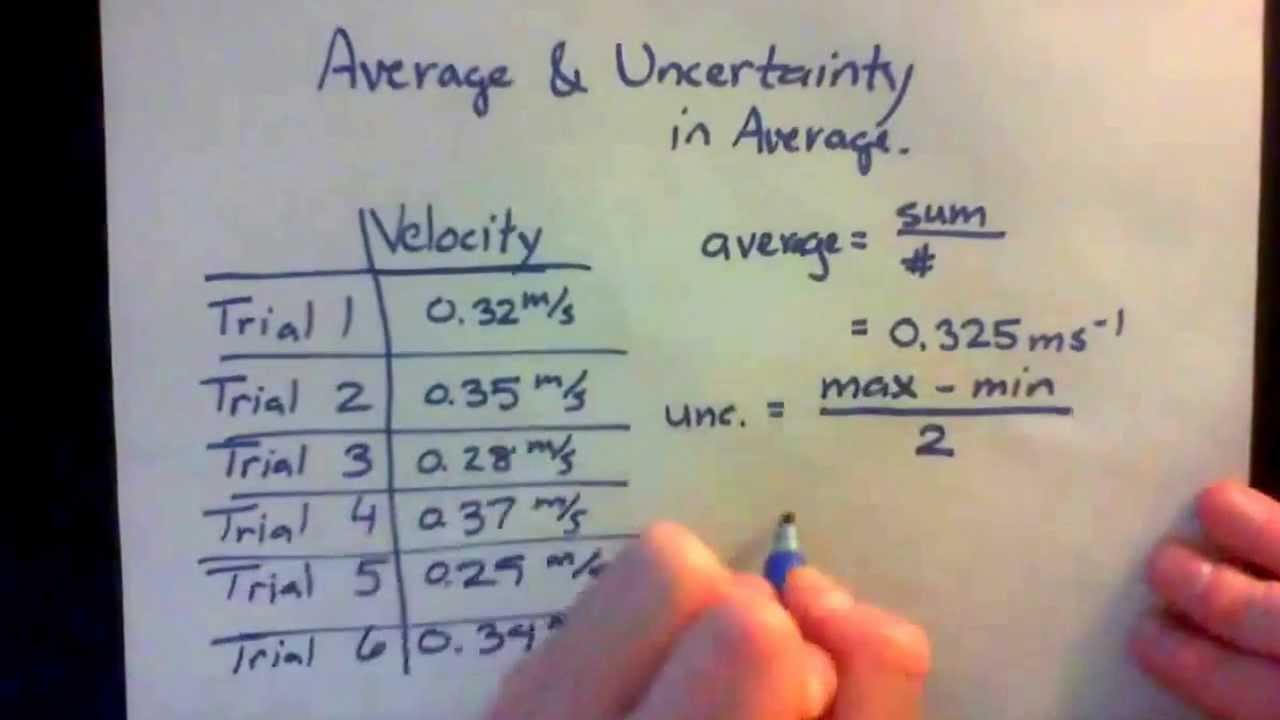
Knowledge that especially for small sample sizes s. Uncertainty measures the lack of certainty or sureness of an outcome. Your Uncertainty science formula picture are available. Uncertainty science formula are a topic that is being hunted for and liked by netizens now. You can Download or bookmark the Uncertainty science formula files here
Uncertainty science formula - It was found that the uncertainty of the model was. Given measurements are v 40ms m 9110 31 kg h 662610 34 6. Solved Examples for Heisenberg Uncertainty Formula. In other words uncertainty in science refers to the idea that all data have a range of expected values as opposed to a precise point value.
Measurement uncertainties can come from the measuring instrument from the item being measured from the environment from the operator and from other sources. 54 67 38 51 63. In other words uncertainty in science refers to the idea that all data have a range of expected values as opposed to a precise point value. The formula for Heisenberg Uncertainty principle is articulated as Where.
Every measurement is subject to some uncertainty. The number of measurements is N5 so the mean is. This uncertainty can be categorized in two ways. Uncertainty of Measurements Measurements are quantified by associating them with an uncertainty.
To express this sense of precision you need to calculate the percentage uncertainty. 67 5462 15376. The relative uncertainty or relative error formula is used to calculate the uncertainty of a measurement compared to the size of the measurement. It is calculated as.
The result of position and momentum is at all times greater than h4π. Is the uncertainty in position. This particular single choice is usually called the measured value which may be optimal in some well-defined sense eg a mean median or mode. It arises in any number of fields including insurance philosophy.
Is 259cm but due to uncertainty the length might be as small as 257cm or as large as 261cm. The formula for Heisenberg Uncertainty principle is given mathematically as. The standard uncertainty that is often associated with such average as estimate of. Uncertainty half the range frac202 cm 3 10 cm 3.
It is calculated as. Showing uncertainty on a graph. For example the best estimate of a length. Uncertainty of a measured value can also be presented as a percent or as a simple ratio.
The uncertainty in the momentum Δp of the electron is 10 6 31 kg using Heisenberg Uncertainty Formula. If youre taking the power of a number with an uncertainty you multiply the relative uncertainty by the number in the power. Uncertainty arises in partially observable or stochastic environments as well as due to ignorance indolence or both. Is a rather unreliable evaluation of.
If one of the uncertainty terms is more than 3 times greater than the other terms the root-squares formula can be skipped and the combined uncertainty is simply the largest uncertainty. Therefore only γ-HCH is discussed here. Your measurement of the table is very precise but your measurement of the width of the hair is rather crude. The uncertainty principle says that both the position and momentum of a particle cannot be determined at the same time and accurately.
A measurement result is only complete if it is accompanied by a statement of the uncertainty in the measurement. Results of uncertainty analysis by basic Monte Carlo method for α-HCH were similar to those for γ-HCH. The following measurements are given. Clearly you know more about the length of the table than the width of the hair.
1 An electron in a molecule travels at a speed of 40ms. So the volume is 240 cm 3 10 cm 3. We know that P mv. The model uncertainty for each phase is illustrated in terms of the semi-interquartile range ie the range between 25th and 75th percentiles by dashed lines in Figure 313.
54 5462 00036. Uncertainty refers to epistemic situations involving imperfect or unknown information. Calculate the average value of all the measurements. When calculating percent uncertainty absolute uncertainty is used.
Formula to calculate percent uncertainty. Relative uncertainty absolute error measured value. Scientific uncertainty is a quantitative measurement of variability in the data. To do this divide the.
Denotes the standard deviation of the observations. Calculate the deviation of each measurement which is the absolute value of the difference between each measurement and the average value. 54 67 38 51 63 5 546. Is the uncertainty in momentum.
Plancks constant having value 6626 times 10 -34 Js. Scientific uncertainty is a quantitative measurement of variability in the data. 152 deviation measurement average. Can be expressed with its uncertainty in two different ways.
Uncertainty can also be shown on a graph. Uncertainty analysis addresses fidelity and is used in different phases of an experiment from initial planning to final reporting Attention is needed to ensure uncertainties do not invalidate your efforts In propagating uncorrelated errors from individual measurement to final result use the square root of the sums of the squares of the. U µ because there is considerable uncertainty associated with. This shortcut can save a lot of time without losing any accuracy in the estimate of the overall uncertainty.
Thus the relative measurement uncertainty is the measurement uncertainty divided by the absolute value of the measured value when the measured value is not zero. 151 average sum of measurements number of measurements. Estimating the Uncertainty in Measurements Before you combine or do anything with your uncertainty you have to determine the uncertainty in your original measurement. Calculate the mean of all the measurements.
However it is common. P 9110 31 40 36410 31 kgms.
Source Image @ www.wikihow.com
Source Image @ www.youtube.com
Source Image @ www.wikihow.com
Source Image @ www.wikihow.com
Source Image @ www.wikihow.com
Source Image @ www.wikihow.com
Source Image @ www.wikihow.com
Source Image @ www.youtube.com
Source Image @ www.youtube.com
If you are looking for Uncertainty Science Formula you've come to the ideal place. We have 10 graphics about uncertainty science formula including images, photos, photographs, wallpapers, and more. In such web page, we also have variety of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
If the posting of this site is beneficial to our suport by expressing article posts of this site to social media accounts as such as for example Facebook, Instagram among others or can also bookmark this blog page along with the title Uncertainties Physics A Level Gcse Youtube Make use of Ctrl + D for pc devices with Home windows operating-system or Demand + D for personal computer devices with operating system from Apple. If you use a smartphone, you can also use the drawer menu on the browser you utilize. Whether its a Windows, Apple pc, iOs or Google android operating-system, you'll be in a position to download images utilizing the download button.








0 comments:
Post a Comment