Science gas formula ~ See our module The Mole. The present discussion focuses on dilute ideal gases in which. Indeed lately is being hunted by users around us, maybe one of you. Individuals are now accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the title of the article I will discuss about Science Gas Formula Gas volume of Z moles of Z x volume of 1 mole.
Source Image @ in.pinterest.com
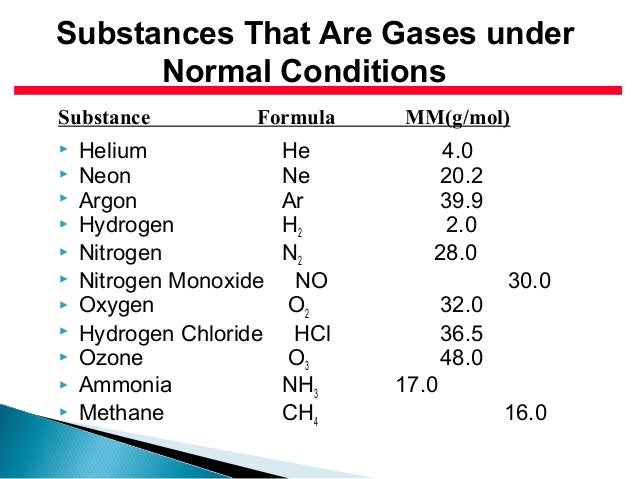
Chemical Formula Science Notes Chemistry Lessons Teaching Chemistry
Therefore an alternative form of the ideal gas law may be useful. It can be used to calculate any of the four properties if the other three are known. Your Science gas formula photographs are ready. Science gas formula are a topic that has been hunted for and liked by netizens today. You can Get or bookmark the Science gas formula files here
Science gas formula - Maximum change of velocity of the vehicle. Kinetic Theory of Gas Ideal Gas Law. With the addition of Avogadros law the combined gas law develops into the ideal gas law. Type of reaction Generalized formula Specific Example Combustion HC O 2 H 2 O CO 2 2C 2 H 6 7O 2 6H 2.
The ideal gas law is derived from empirical relationships among the pressure the volume the temperature and the number of moles of a gas. The ideal gas law PV nRT Worked example. It is a non-metallic element that occupies Group 15 of the periodic table. T Absolute temperature of the gas.
Where P Pressure of the gas. The formula explains the motion of vehicles based on acceleration and using its thrust to get high velocity which is on the basis of conservation of momentum. N 12 l 10 2 cm. The equation of state for an ideal or perfect gas is the ideal gas law and reads P V n R T displaystyle PVnRT where P is the pressure V is the volume n is amount of gas in mol units R is the universal gas constant 8314 Jmol K and T is the temperature.
Displaystyle n frac m M. Over the past four centuries scientists have performed many experiments to understand the common behaviors of gases. In the case of real gas. The equation is given as.
The Combined gas law or General Gas Equation is obtained by combining Boyles Law Charless law and Gay-Lussacs Law. Gas - gas - Kinetic theory of gases. The general equation of combined gas law is given as. Δv velnm0 mf I spg0lnm0 mf Δ v v e l n m 0 m f I s p g 0 l n m 0 m f.
C 6 H 14. If we want to compare the same gas in different cases the law can be represented as. Gas molecules move in three dimensions whereas the drunkard moves in two dimensions. The aim of kinetic theory is to account for the properties of gases in terms of the forces between the molecules assuming that their motions are described by the laws of mechanics usually classical Newtonian mechanics although quantum mechanics is needed in some cases.
Nitrogen gas has two molecules of Nitrogen therefore the molecular formula of this gas is N2. 1164V nRTZ P dV dP. The equation can be rearranged to find the number of moles if the volume of gas at rtp is known. They have observed that a gass physical conditionits statedepends on four variables.
There are 500 moles of gas molecules in a container. 1 mole formula mass of Z in g. If 220 K temperature is applied to the gas of a volume of 40 L identify the Gas pressure. Moles Z mass of Z gas g atomic or formula mass of gas Z gmol mass of Zin g moles of Zx atomic or formula mass of Z.
Much like the combined gas law the ideal gas law is also an amalgamation of four different gas laws. Its History and Use for more information. Gas mixtures and partial pressures. The chemical amount n in moles is equal to total mass of the gas m in kilograms divided by the molar mass M in kilograms per mole.
The isothermal gas compressibility is defined as the change in relative volume per unit pressure drop at a constant temperature. It shows the relationship between the pressure volume and temperature for a fixed mass quantity of gas. The Ideal gas pressure formula is given as Where V volume n number of moles R Gas constant 83145 JolmolK T temperature. Number of moles volume of gas at rtp 24.
Cg is the gas compressibility 1 psi. However the result is the same. The ideal gas equation is. PV T k.
Using the above relation and knowing the remaining quantities the molecular mass can be calculated. Nitrogen gas is colorless odorless and tasteless which was discovered by a well-known scientist named Daniel Rutherford in. CHO 3 120. Equation 3223 is the gas material balance and it shows that a plot of Pz versus gas production is linear Figure 321.
Equation 3224 shows that the x-intercept of a Pz plot is equal to the original gas in place in surface volumes. V Volume of the gas. R Universal gas constant. N Number of moles of the gas.
The SI unit for Gas pressure is expressed in Pascals Pa. Pressure P volume V temperature T and amount n in moles. 1163C g 1 V dV dPT. P 1 V 1 T 1 P 2 V 2 T 2 Also Read.
Thus the square root of N multiplied by the length of the mean free path equals the length of the diffusion tube. Using the ideal gas law to calculate number of moles. ScienceChemistry libraryGases and kinetic molecular theoryIdeal gas equation. Atomic or formula mass of Z mass of Z moles of Z.
H 2 O 2. H 3 PO 4. PV nRT where R 008206L atm K mol 83145 J K mol. Pressure Increasing pressure favors the reaction that produces less gas.
Using the ideal gas law to calculate a change in volume. Gasoline ˈ ɡ æ s ə l iː n or petrol ˈ p ɛ t r ə l see the etymology for naming differences and the use of the term gas is a transparent petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion enginesIt consists mostly of organic compounds obtained by the fractional distillation of petroleum enhanced with a variety. This can also be written as. N m M.
Source Image @ www.slideshare.net
Source Image @ www.youtube.com
Source Image @ slideplayer.com
Source Image @ www.youtube.com
Source Image @ www.pinterest.fr
Source Image @ knowtobank.blogspot.com
Source Image @ www.youtube.com
Source Image @ en.wikipedia.org
Source Image @ www.pinterest.com
If you re searching for Science Gas Formula you've arrived at the perfect place. We have 10 images about science gas formula including pictures, photos, photographs, wallpapers, and much more. In such webpage, we also have number of graphics available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
If the posting of this website is beneficial to your suport by sharing article posts of the site to social media marketing accounts you have such as Facebook, Instagram and others or can also bookmark this blog page using the title Chemistry Lessons Chemistry Classroom Science Notes Use Ctrl + D for pc devices with Home windows operating system or Order + D for laptop devices with operating system from Apple. If you use a smartphone, you can also use the drawer menu of the browser you use. Be it a Windows, Mac, iOs or Android os operating system, you'll still be able to download images using the download button.









0 comments:
Post a Comment