Planck's quantum theory formula ~ Class 11 Chemistry Plancks quantum theory. In metrology it is used together with other. Indeed lately is being searched by consumers around us, perhaps one of you personally. People now are accustomed to using the net in gadgets to view video and image data for inspiration, and according to the title of this post I will discuss about Planck's Quantum Theory Formula Recently came across Plancks theory E h ν.
Source Image @ www.youtube.com
Chemistry Planck S Quantum Theory Of Radiation Structure Of Atom Part 5 English Youtube
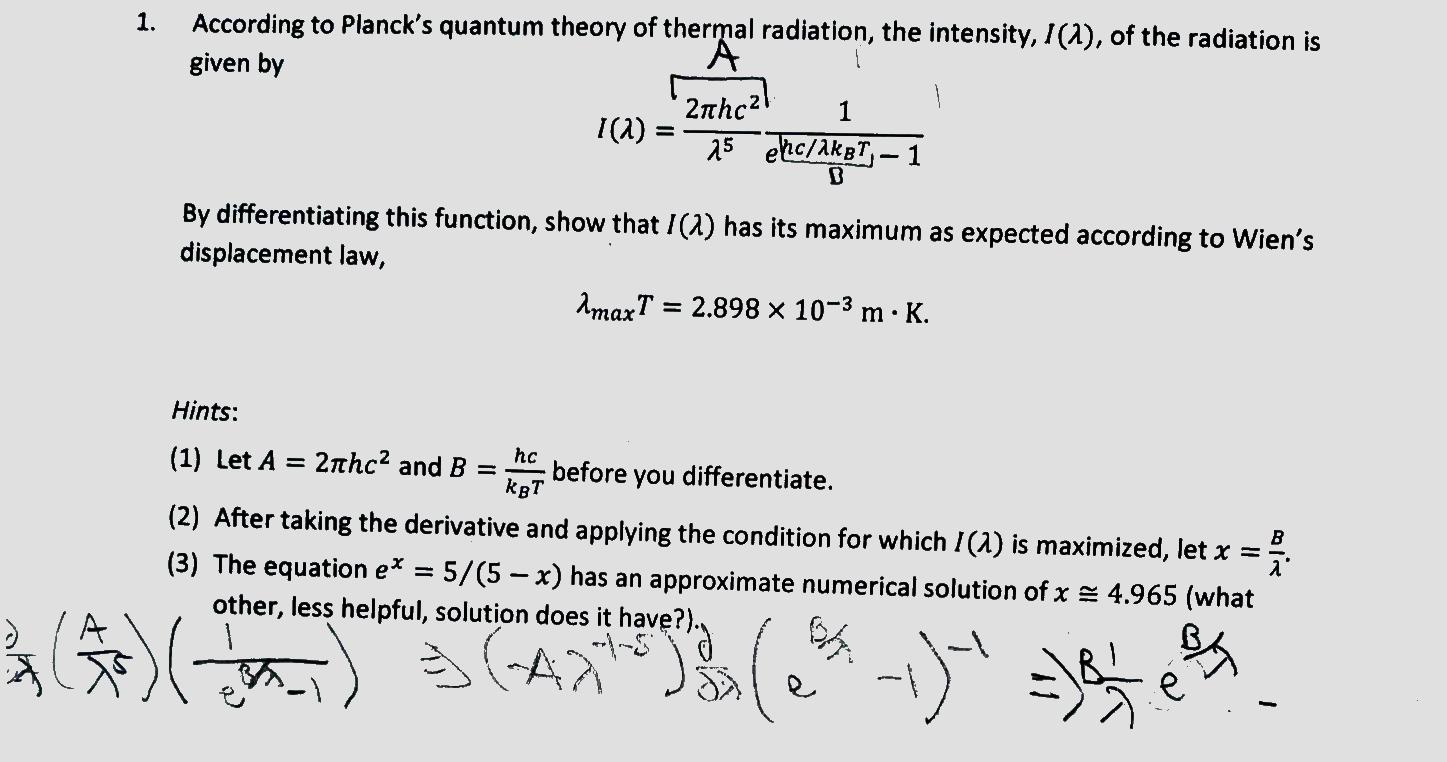
In 1899-1900 Max Planck created quantum mechanics with his discovery that light maintains thermodynamic equilibrium between matter and an equilibrium radiation field satisfying the distribution law he disclosed in the same 1900 paper through tiny prolonged energy transfers Ehf he called quanta occurring at the light-matter interface. Pler formula is given by vv2 1 cos1ETE 7 From equation 6 and 7 we finally get EvEvh invariant We get Plancks relation Eh Qwhere h is inertial frame invariant. Your Planck's quantum theory formula image are available in this site. Planck's quantum theory formula are a topic that is being searched for and liked by netizens now. You can Download or bookmark the Planck's quantum theory formula files here
Planck's quantum theory formula - Eh nu labelEq121 where the proportionality constant h is called Plancks constant one of the most accurately known fundamental constants in science h662607004081 times 1034 Jcdot s nonumber. To explain these radiations Max Planck put forward a theory known as Plancks quantum theory. I Substances radiate or absorb energy discontinuously in the form. It was Planck however who started it off by introducing his quantum of action the Planck constant h.
A photons energy is equal to its frequency multiplied by the Planck constant. In 1900 Planck identified his game-changing constant by describing how the smallest bits of matter release energy in discrete bundles called quanta essentially placing the quanta in quantum mechanics. To explain the colors of hot glowing matter he proposed that energy is radiated in very minute and discrete quantized amounts of packets rather than in a continuous unbroken wave. E depends upon v nu.
Energy emitted or absorbed is not continuous but is in the form of packets called quanta In terms of light it is called as photon. According to the theory of relativity a particle of mass m has equivalent energy Emc 2 where c is the speed of light. Due to massenergy equivalence the Planck constant also relates mass to frequency. Now v 310 8 380 10-9 1 nm 10-9 m Hence v 78910 14 s.
Moller gave a very cumbrous proof 1 of the in-variance of h using plane monochromatic wave function in a lengthy and complicated 4 dimensional formalism and added. The constants C 1 and C 2 are numbers chosen by Planck to make the equation fit the experiments. Among those present at the historic seminar was. Planck tried to alter his expression for the entropy of the radiation by generalizing it and eventually arrived at a new formula for the radiation intensity over the entire frequency range.
It has dimensions of power per solid angle per area per frequency or power per solid angle per area per wavelength. According to quantum theory a photon of electromagnetic radiation of frequency ν has energy Ehν where h is Plancks constant. Plancks work in thermodynamics led to the formulations of his quantum theory. The Planck constant or Plancks constant is a fundamental physical constant denoted h displaystyle h and is of fundamental importance in quantum mechanics.
Plancks quantization of energy is described by the his famous equation. The main points of quantum theory are. Plancks quantization of energy is described by his famous equation. According to this theory.
Show activity on this post. Plancks constant is currently calculated by scientists to be 662607015 x 10-34 joule-seconds. Einstein would later use h to calculate the energy of a photon as E phohf wherein the energy of a photon E pho equals Plancks constant h times the photons frequency f. To find the Energy we know that E hν.
F is the equilibrium radiation. But I also saw that E can be 0 h ν 2 h ν 3 h ν. Planck said that the energy of a quantum is proportional to the frequency of the radiation according to the equation Equantumhvradiation. To find the Frequency the formula used is c λv.
It means that at any frequency there is given energy. V represents the frequency of light. Mathematically B_lambda Tfrac2hc2lambda 5frac1efrachckTlambda -1. Or Ehv where v is frequency.
When a black body is heated it emits thermal radiation of different wavelengths or frequency. Where c represents the speed of light. Where h represents the plancks constant. At at given frequency there should be certain energy only.
λ represents the wavelength of violet light. Each photon carries an energy which is directly proportional to the frequency of wavelength ie. It states that electromagnetic radiation from heated bodies is not emitted as a continuous flow but is made up of discrete units or quanta of energy the size of which involve a fundamental physical constant Plancks constant. Plancks formula for the distribution of energy in the radiation from a black body was the starting point of the quantum theory which has been developed during the last 20 years and has borne a wealth of fruit in the energy domain of physics.
Plancks law is a formula for the spectral radiance of an object at a given temperature as a function of frequency Lf or wavelength Lλ. How is it possible that energy can be varied at any given frequency.
Source Image @ www.facebook.com
Source Image @ www.brainkart.com
Source Image @ www.youtube.com
Source Image @ pt.slideshare.net
Source Image @ www.quora.com
Source Image @ oxscience.com
Source Image @ byjus.com
Source Image @ www.slideshare.net
Source Image @ www.chegg.com
If you are looking for Planck's Quantum Theory Formula you've arrived at the perfect place. We have 10 images about planck's quantum theory formula including images, photos, pictures, wallpapers, and much more. In these page, we also have variety of graphics available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
If the posting of this webpage is beneficial to your suport by sharing article posts of this site to social media accounts which you have such as Facebook, Instagram and others or can also bookmark this blog page with all the title Solved 1 According To Planck S Quantum Theory Of Thermal Chegg Com Use Ctrl + D for personal computer devices with Windows operating-system or Order + D for computer system devices with operating system from Apple. If you are using a smartphone, you can also use the drawer menu of this browser you utilize. Whether its a Windows, Apple pc, iOs or Android os operating-system, you'll still be able to download images utilizing the download button.


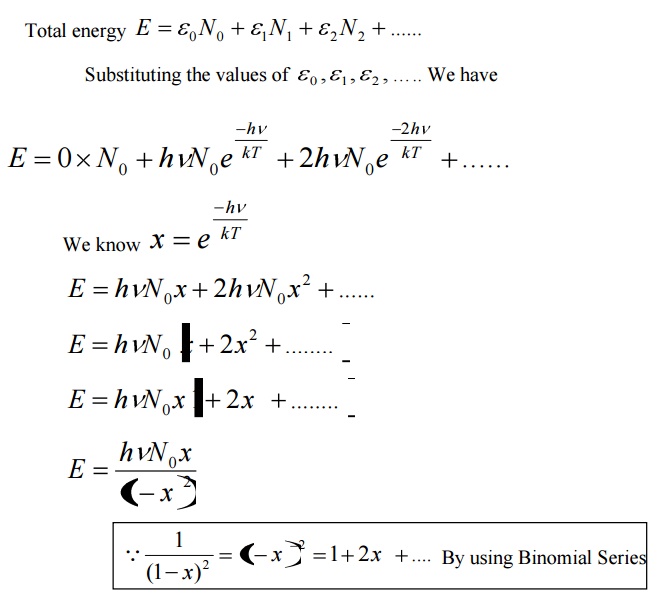

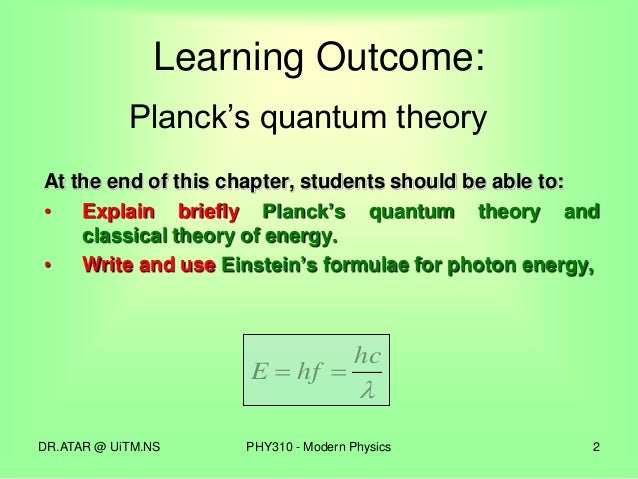
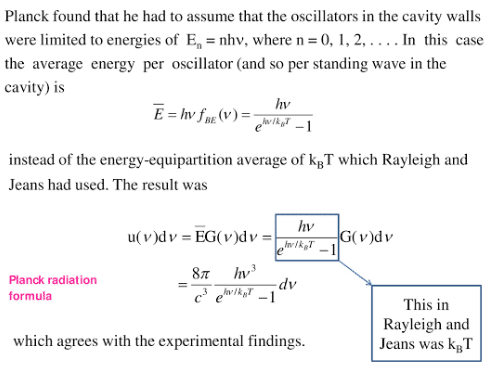


0 comments:
Post a Comment