Orbital quantum number formula ~ Since each set is unique they serve as a way of uniquely naming individual electrons ie. Note This formula is not applicable to f- block elements. Indeed lately is being searched by users around us, maybe one of you personally. People now are accustomed to using the net in gadgets to see image and video data for inspiration, and according to the title of this article I will talk about about Orbital Quantum Number Formula There are 2l1 orbitals in each subshell.
Source Image @ study.com
Magnetic Quantum Number Definition Example Video Lesson Transcript Study Com
For p-orbita ℓ 1. Answer 1 of 2. Your Orbital quantum number formula picture are ready in this website. Orbital quantum number formula are a topic that has been hunted for and liked by netizens now. You can Download or bookmark the Orbital quantum number formula files here
Orbital quantum number formula - Calculate the minimum and maximum number of electrons which have magnetic quantum number m 1 and spin quantum number s 12 in chromium. L 2 Y ℓ m θ ϕ ℏ 2 ℓ ℓ 1 Y ℓ m θ ϕ The spherical. The number of orbitals in a shell is the square of the principal quantum number. Magnetic quantum number ml 2 1 0 1 2.
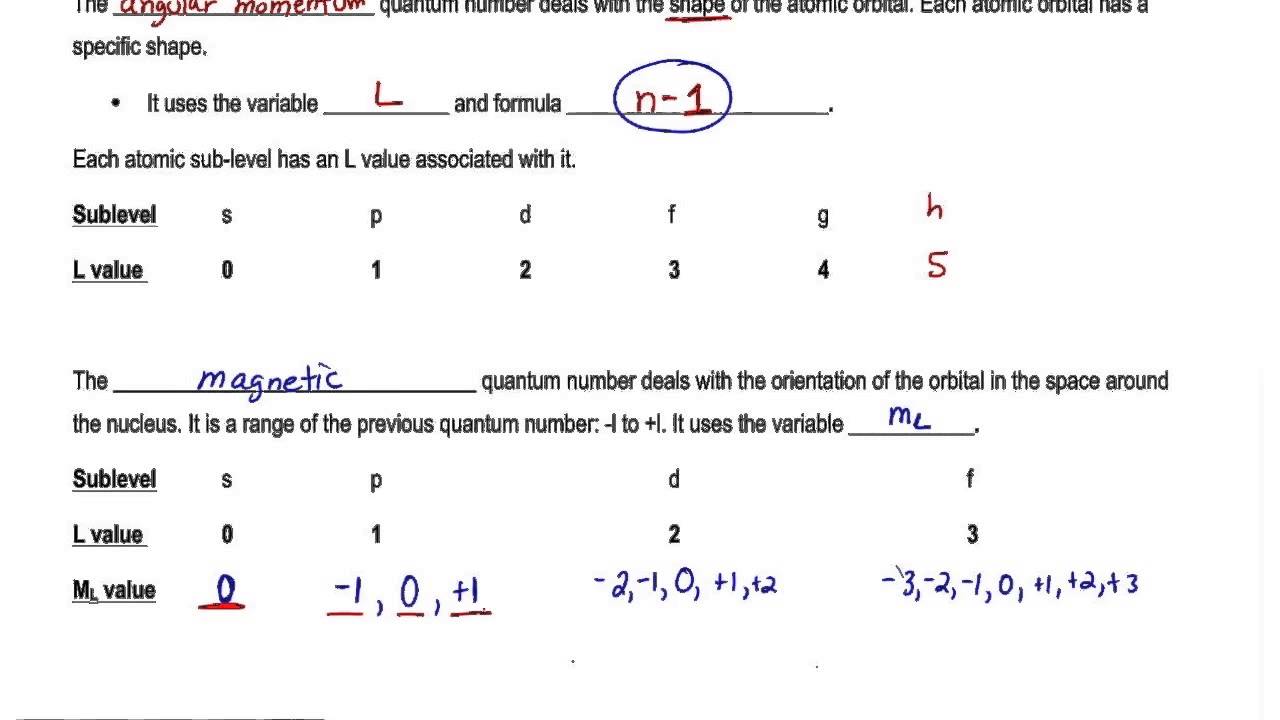
How many possible numbers of orbitals of an atom when the principal quantum number equal to four. The number l called the orbital quantum number must be less than the principal quantum number n which corresponds to a shell of electrons. So the orbital angular momentum quantum number is 3-1 that is 2. For s-orbital ℓ 0.
Azimuthal quantum number l 2. For f-orbital ℓ 3. Orbital magnetic moment This magnetic moment is due to the orbital motion It may be calculated by the formula Orbital magnetic moment l l 1 h 2 π. It determines the orientation of the orbital in space relative to the other orbitals in the atom.
With the help of the value of azimuthal quantum number we can determine the total number of energy sub-levels in a given energy level. The number of shell represents the principal quantum number. Other articles where orbital quantum number is discussed. The number of angular nodes is equal to the value of the angular momentum quantum number l.
A set of the four quantum numbers describes the unique properties of one specific electron in an atom. Each orbital can accommodate 2 electrons so there will be a total of 14 or 7 2 electrons in 3f subshell. This quantum number has values from -ℓ through zero to ℓ. The orbital quantum number arises when solving the Schrodingers equation.
In this n is principal quantum number. Specifies the orientation in space of an orbital of a given energy n and shape l. What is the formula of principal quantum number. It is denoted by.
The Orbital Angular Momentum Quantum Number l The orbital angular momentum quantum number l determines the shape of an orbital and therefore the angular distribution. Orbital velocity is velocity obtained by movement of electrons around nucleus. Numbers of possible orbitals when principle quantum number equal to four 1 4s 3 4p 5 4d 7 4f 16. The number of orbitals in a subshell is therefore 2l 1.
L 3 m 3 2 1 0-1 -2 -3 the orbital is f The number of value of m determines the number of orbital of a particular type. Angular momentum quantum number l n 1. The values of ℓ are the eigenvalues of the angular momentum operator squared and the spherical harmonics Y are its eigenfunctions. Values of are from zero to n-1.
This number divides the subshell into individual orbitals which hold the electrons. The orbital angular momentum is given by n1 formula. The first three n n n ℓ ell ℓ and m ℓ m_ell m ℓ come from the solution to the spherical Schrödinger equation and describe the orbital of the electron. A kind of coordinate system.
Magnetic Quantum Number m l. Spin can either be 12 or -12. M l -l 0 l. Azimuthal quantum number describes the shape of orbital.
The possible total number of orbitals in a given subshell for n 4 and ℓ 3 is 23 1 7. Each l value represents a specific orbit named after the description of the hydrogen spectrum such s for sharp l 0 p for principal l 1 d for diffuse l 2 f for fundamental l 3 etc. Thus the s subshell has only one orbital the p subshell has three orbitals and so on. L azimuthal quantum number of orbital.
For s orbital l0. There is one orbital in an ssubshell l 0 three orbitals in a psubshell l 1 and five orbitals in a dsubshell l 2. Angular momentum quantum numbers. Specifies which orbital within a sublevel you are likely to find the electron.
The values of magnetic quantum numbers will be -3 -2 -1 0 1 2 and 3. Spin quantum number ms ½ or -½. Magnetic quantum number mℓ-2 -1 0 1 2. Electron has two types of velocity orbital velocity and axial velocity.
Magnetic quantum number m l l1 l2. 12 1 22 4 32 9. Spin quantum number s. For d-orbital ℓ 2.
Thus l divides each shell into n subshells consisting of all electrons of the same principal and orbital quantum numbers. For more information about angular nodes see Electronic Orbitals Each value of l indicates a specific. So the value of principal quantum number for 3d is 3. For the 3d orbital Principal quantum number n 3.
Source Image @ www.priyamstudycentre.com
Source Image @ www.youtube.com
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ www.khanacademy.org
Source Image @ study.com
Source Image @ slideplayer.com
Source Image @ slideplayer.com
Source Image @ www.pinterest.com
Source Image @ www.youtube.com
If you re searching for Orbital Quantum Number Formula you've reached the perfect location. We have 10 images about orbital quantum number formula adding pictures, pictures, photos, wallpapers, and much more. In these webpage, we also have variety of images out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, translucent, etc.
If the publishing of this webpage is beneficial to our suport by sharing article posts of this site to social media marketing accounts that you have got such as for example Facebook, Instagram and others or can also bookmark this blog page while using title Solving The Schrodinger Equation Youtube Make use of Ctrl + D for laptop devices with Windows operating system or Command word + D for pc devices with operating-system from Apple. If you use a smartphone, you can also utilize the drawer menu with the browser you utilize. Whether its a Windows, Apple pc, iOs or Google android operating-system, you'll still be able to download images using the download button.








0 comments:
Post a Comment