Quantum wavelength formula ~ Schrödingers equation can be solved to yield a series of wave function each of which is associated with an electron binding energy. Quantum Mechanics Formulas. Indeed recently has been hunted by consumers around us, maybe one of you personally. Individuals are now accustomed to using the internet in gadgets to see video and image information for inspiration, and according to the title of this post I will talk about about Quantum Wavelength Formula Erwin Schrödinger proposed the quantum mechanical model of the atom which treats electrons as matter waves.
Source Image @ www.youtube.com
Physics Ch 66 Quantum Mechanics 2 Basic Concepts 9 Of 38 The Compton Wavelength Youtube
The wavelength formula is given by λ v f. According to Plancks theory every quantum of a wave has a discrete amount of energy associated with it and he gave the equation. Your Quantum wavelength formula pictures are ready in this website. Quantum wavelength formula are a topic that has been searched for and liked by netizens now. You can Get or bookmark the Quantum wavelength formula files here
Quantum wavelength formula - The following equation is used to calculate a de broglie wavelength. Here m v is the momentum p of the particle. Wavelength can be assumed to be absorbing the same number of photons. W 1 RZ2 1nf2 1ni2 Where W is the wavelength.
After this the wave equation gets solved to find these modes then the electron gets the energy by the frequency of the model and by Einsteins Quantum Equation that is E hv. Important Formula of Quantum Mechanics. De-Broglie wavelength determines the interference ability of particles. This is useful for.
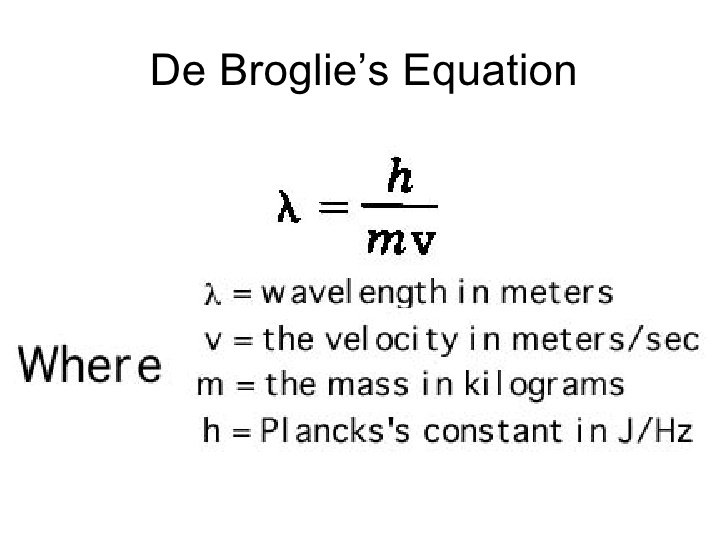
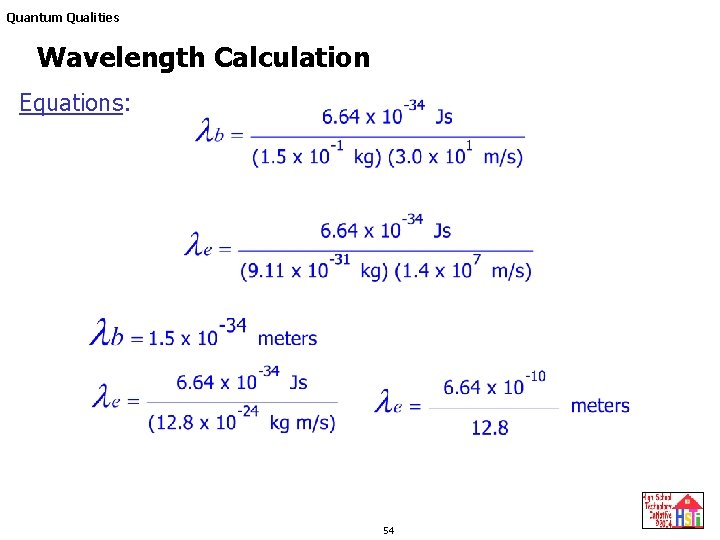
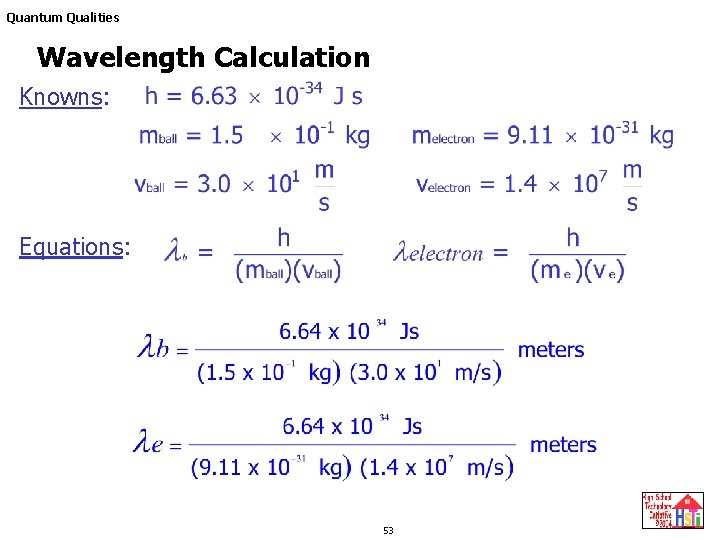
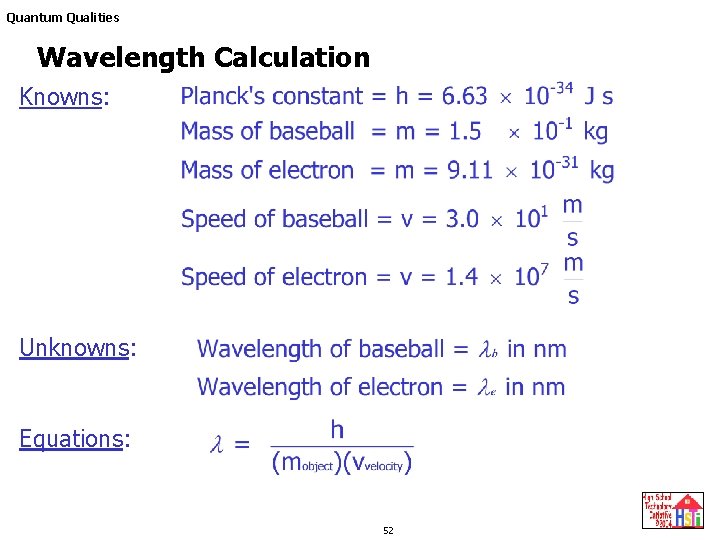
De Broglie wavelength λ De Broglie wavelength λ is the wavelength of these material waves where E is the energy of the particle. According to wave-particle duality the De Broglie wavelength is a wavelength manifested in all the objects in quantum mechanics which determines the probability density of finding the object at a given point of the configuration spaceThe de Broglie wavelength of a particle is inversely proportional to its momentum. L h mv Where L is the wavelength. E 6626 x 10 -34 Js x 3 x 10 8 msec 633 nm x 10 -9 m1 nm.
Determine the wavelength of sound. The three prominent hydrogen lines are shown at the right of the image through a 600 linesmm diffraction grating. The following formula is used by the calculator to calculate the wavelength of an emitted light. To calculate wavelength use the formula wavelength speed divided by frequency.
Every particle of mass m eg. Nf is the principal quantum number in the final state. The wave travels a distance of 3m in 4s. λ 03 m.
The different energy levels of atoms were identified with the simple vibrational modes of the wave equation. The wave speed is calculated by v 3 4. The radius of the Quantum Dot influence the wavelength of the emitted light due to quantum confinement this equation describes the effect of a change of the radius of the quantum dot on the wavelength λ of the emitted light and hence on the emission energy E hc λ where c is the speed of light. Thus from the relation between linear momentum p and energy E of the particle we knew that pEover c.
TextE texthf cdots 2 Where. Remember to use the correct units when youre using the formula and writing your answer. Since ΦF for the standard sample is known it is trivial to calculate the ΦF for the test sample. λ is the wavelength of the photon wavenumber 1wavelength R Rydbergs constant 10973731568539 55 x 10 7 m -1 Z atomic number of the atom.
It was later found that n 2 and n 1 were related to the principal quantum number or energy quantum number. Hence a simple ratio of the integrated fluorescence intensities of the two solutions recorded under identical conditions will yield the ratio of the quantum yield values. Just plug in the waves speed and frequency to solve for the wavelength. In other words the energy of a photo is directly proportional to its frequency and inversely proportional to its wavelength.
N 1 and n 2 are integers where n 2 n 1. H is Planks constant 66262 X 10 -34 Js m is the mass grams v is the velocity ms De Broglie Wavelength Definition. The frequency of a tuning fork is 200 Hz and sound travels a distance of 20 m while tuning fork executes 30 vibrations. Electron proton can be assigned a wavelength lambda in quantum mechanics the so-called matter wavelength also called De-Broglie wavelength.
All that remains is to plug in the values and get the answer. E hν. And quantum energy hν eV At left is a hydrogen spectral tube excited by a 5000 volt transformer. De Broglie Wavelength Formula.
R is the rydberg constant 1097 107 m-1 Z is the number of protons in the nucleus of the element.
Source Image @ www.toppr.com
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ hyperphysics.phy-astr.gsu.edu
Source Image @ www.sliderbase.com
Source Image @ www.a-levelphysicstutor.com
Source Image @ slidetodoc.com
Source Image @ slidetodoc.com
Source Image @ slidetodoc.com
Source Image @ www.quora.com
If you re looking for Quantum Wavelength Formula you've arrived at the right place. We ve got 10 graphics about quantum wavelength formula including pictures, pictures, photos, wallpapers, and much more. In these webpage, we additionally have number of images available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.
If the posting of this internet site is beneficial to our suport by expressing article posts of the site to social media marketing accounts which you have such as Facebook, Instagram among others or may also bookmark this blog page while using title What Is De Broglie Wavelength Quora Work with Ctrl + D for computer system devices with Home windows operating system or Order + D for laptop devices with operating-system from Apple. If you use a smartphone, you can also use the drawer menu from the browser you use. Be it a Windows, Mac, iOs or Google android operating system, you'll be in a position to download images utilizing the download button.

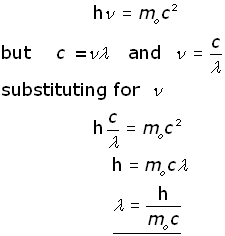
0 comments:
Post a Comment